Calculations of thermal effects of reactions. Methods for calculating the thermal effects of chemical reactions at different temperatures
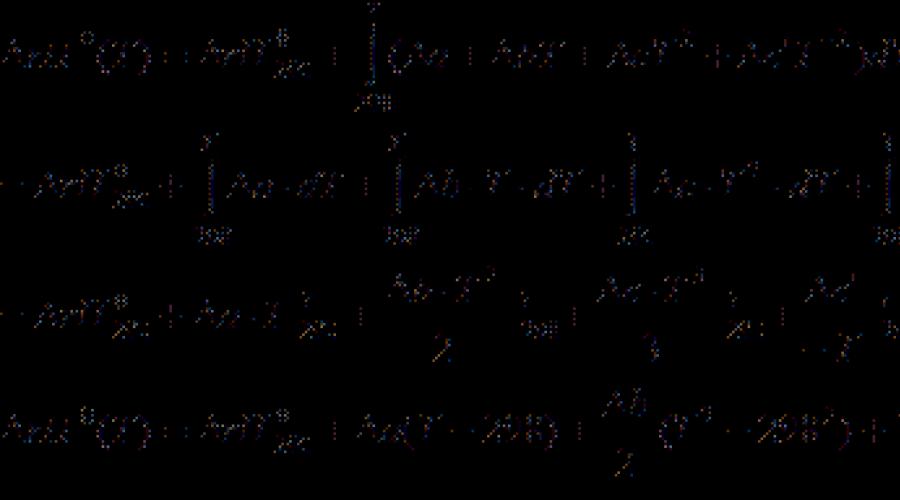
Read also
All methods for calculating thermal effects are based on the Kirchoff equation in the integral form.
Most often, standard 298.15K is used as the first temperature.
All methods for calculating thermal effects are reduced to methods of taking the integral of the right part of the equation.
Integral Taking Methods:
I. Middle heat caps. This method is the easiest and most accurate. In this case, the expression under the integral sign is replaced by the change in the average heat capacity, which does not depend on the temperature in the selected range.
The average heat capacity is tabulated and measured for most reactions. They are easy to calculate on reference data.
II. According to true heat caps. (With the help of temperature rows)
In this method, the integrand expression of heat capacity is written as a temperature range:

III. According to high-temperature components of enthalpy. This method was widely distributed with the development of rocket technology when calculating the thermal effects of chemical reactions at high temperatures. It is based on the definition of isobar heat capacity:

High temperature component of enthalpy. It shows how much the enthalpy of the individual substance will change when it is heated to a certain number of degrees.
For chemical reaction, write:

In this way:
Lecture number 3.
Lecture Plan:
1. II The law of thermodynamics, definition, mathematical record.
2. Analysis of the II of the Law of Thermodynamics
3. Calculation of entropy changes in some processes
here and further indexes i. refer to the initial substances or reagents, and indices j.- to finite substances or reaction products; and - stoichiometric coefficients in the reaction equation for the starting materials and reaction products, respectively.
Example:Calculate the thermal effect of the methanol synthesis reaction under standard conditions.
Decision: For calculations, we will use reference data according to the standard heat of the formation involved in the substances reaction (see Table. 44 on page 72 of the reference book).
The thermal effect of the methanol synthesis reaction under standard conditions under the first consequence of the GESS law (equation 1.15) is equal:
When calculating the thermal effects of chemical reactions, it is necessary to take into account that the thermal effect depends on the aggregate state of the reagents and on the type of recording of the chemical reaction equation:
On the second consequence of the GESSA law, the thermal effect can be calculated using the heat of combustion Δ C H.As the difference in the heat of the heat of the combustion of the starting materials and the reaction products (taking into account the stoichiometric coefficients):
where Δ. R C P. - characterizes the change in the isobaric heat capacity of the system as a result of the flow of a chemical reaction and is called the temperature coefficient of the thermal effect of the reaction.
From the Differential Kirchhoff equation it follows that the dependence of the thermal effect on temperature is determined by the sign Δ R C P.. It depends on the fact that more, the total heat capacity of the initial substances or the total heat capacity of the reaction products. Let us analyze the differential equation of Kirchhoff.
1. If the temperature coefficient Δ R C P.\u003e 0, then derivative  \u003e 0 and function
\u003e 0 and function  increasing. Consequently, the thermal effect of the reaction with increasing temperature increases.
increasing. Consequently, the thermal effect of the reaction with increasing temperature increases.
2. If the temperature coefficient Δ R C P.< 0, то производная  < 0 и функция
< 0 и функция  descending. Consequently, the thermal effect of the reaction with increasing temperature decreases.
descending. Consequently, the thermal effect of the reaction with increasing temperature decreases.
3. If the temperature coefficient Δ R C P. \u003d 0, then derivative  \u003d 0 I.
\u003d 0 I.  . Consequently, the thermal effect of the reaction does not depend on temperature. This case does not occur in practice.
. Consequently, the thermal effect of the reaction does not depend on temperature. This case does not occur in practice.
Differential equations are convenient for analysis, but uncomfortable for calculations. In order to obtain an equation for calculating the thermal effect of a chemical reaction, in cross the differential equation of Kirchhoff, separating the variables:


The heat capacity of substances depend on temperature, therefore, and  . However, in the region of the temperatures commonly used in chemical and technological processes, this dependence is not significant. For practical purposes, medium heat-capacity substances are used in the temperature range from 298 to the specified temperature.
. However, in the region of the temperatures commonly used in chemical and technological processes, this dependence is not significant. For practical purposes, medium heat-capacity substances are used in the temperature range from 298 to the specified temperature.  which are given in reference books. Temperature coefficient of thermal effect, calculated using medium heat capacity:
which are given in reference books. Temperature coefficient of thermal effect, calculated using medium heat capacity:
Example:Calculate the thermal effect of the methanol synthesis reaction at a temperature of 1000 to and standard pressure.
Decision: For calculations, we will use the reference data on the average heat capacity of the substances involved in the reaction in the temperature range from 298 to to 1000 K (see Table 40 on page 56 of the directory):
Changes in the average heat capacity of the system as a result of the flow of a chemical reaction:
The second beginning of the thermodynamics
One of the most important tasks of chemical thermodynamics is to clarify the principal possibility (or the inability) of the spontaneous flow of a chemical reaction in the direction under consideration. In cases where it becomes clear that this chemical interaction may occur, it is necessary to determine the degree of transformation of the source substances and the yield of the reaction products, that is, the fullness of the reaction
The direction of course of the spontaneous process can be determined on the basis of the second law or the onset of thermodynamics formulated, for example, in the form of a Clausius postulate:
The heat itself cannot move from a cold body to hot, that is, such a process is impossible, the only result of which was the transition of heat from the body with a lower temperature to the body with a higher temperature.
A variety of formulations of the second start of thermodynamics are proposed. Thomson's wording - Planck:
The eternal engine of the second kind is impossible, i.e., such a periodically active machine is impossible, which would allow to obtain operation only by cooling the heat source.
The mathematical formulation of the second start of thermodynamics occurs when analyzing the operation of thermal machines in the works of N. Carno and R. Clausius.
Clausius was introduced a state function S., named entropy, the change in which is equal to the heat of the reversible process, referred to temperature
For any process
 | (1.22) |
The resulting expression is a mathematical expression of the second start of thermodynamics.
Standard warmth of education (enthalpy of education) substances It is called the enthalpy of the formation of the formation of 1 praying of this substance from the elements (simple substances, that is, consisting of atoms of one species) in the most stable standard state. Standard environmental enthalpies (CJ / mol) are given in reference books. When using reference values, it is necessary to pay attention to the phase state of substances involved in the reaction. Enthalpy of formation of the most stable simple substances is 0.
Corollary from the GESS law on the calculation of the thermal effects of chemical reactions on the heat of education : standard the thermal effect of the chemical reaction is equal to the heat of the heat of the formation of the reaction products and the heat of the formation of the source substances, taking into account the stoichiometric coefficients (quantities of moles) of the reagents:
Ch 4 + 2 CO \u003d 3 C ( graphite ) + 2 H. 2 O.
gas gas TV. gas
The heat of the formation of substances in these phase states is shown in Table. 1.2.
Table 1.2.
Warm formation of substances
Decision
Since the reaction passes when P.\u003d const, then the standard thermal effect is found as a change in enthalpy according to the known heat of education by consequence of the GESS law (Formula (1.17):
ΔН. about 298 \u003d (2 · (-241.81) + 3 · 0) - (-74.85 + 2 · (-110,53)) \u003d -187,71 kJ \u003d -187710 J.
ΔН. about 298 < 0, реакция является экзотермической, протекает с выделением теплоты.
Change in internal energy we find on the basis of equation (1.16):
ΔU. about 298 = Δh. about 298 – Δ ν · rt..
For this reaction of changes in the number of moles of gaseous substances due to the passage of a chemical reaction Δν = 2 – (1 + 2) = –1; T.\u003d 298 K, then
Δ U. about 298 \u003d -187710 - (-1) · 8,314 · 298 \u003d -185232 J.
Calculation of the standard thermal effects of chemical reactions according to the standard heat of the combustion of substances involved in the reaction
Standard heat combustion (combustion enthalpy) substances
it is called the thermal effect of complete oxidation of 1 praying of a given substance (up to higher oxides or specially indicated compounds) with oxygen, provided that the initial and finite substances have a standard temperature. Standard combustion enthalpia substances  (KJ / mol) are given in reference books. When using reference values, it is necessary to pay attention to the sign of the elephant of the combustion reaction, which is always exothermic ( Δ
H.
<0), а в таблицах указаны величины
(KJ / mol) are given in reference books. When using reference values, it is necessary to pay attention to the sign of the elephant of the combustion reaction, which is always exothermic ( Δ
H.
<0), а в таблицах указаны величины
 .
. Enthalpy combustion of higher oxides (for example, water and carbon dioxide) are equal to 0.
Enthalpy combustion of higher oxides (for example, water and carbon dioxide) are equal to 0.
Corollary from the GESS law on the calculation of the thermal effects of chemical reactions on heat of combustion : the standard thermal effect of the chemical reaction is equal to the heat of the heat of combustion of the initial substances and the heat of the combustion of the reaction products, taking into account the stoichiometric coefficients (quantity of moles) of the reagents:
C. 2 H. 4 + H. 2 O. \u003d S. 2 N. 5 IS HE.
Just as one of the physical characteristics of a person is physical force, the most important characteristic of any chemical communication is the power of communication, i.e. Her energy.
Recall that the chemical bond energy - this energy that is allocated in the formation of a chemical bond or the energy that needs to be spent to destroy this connection.
Chemical reaction in general is the conversion of one substances into others. Consequently, during the chemical reaction, there is a breaking of some connections and the formation of others, i.e. Turning energy.
The fundamental law of physics states that energy does not arise from nothing and does not disappear without a trace, but only passes from one species to another. By virtue of its universality, this principle is obviously applicable to a chemical reaction.
Thermal effect of chemical reaction called the amount of heat,
allocated (or absorbed) during the reaction and the relative to 1 mol reacted (or the resulting) substance.
The thermal effect is indicated by the letter Q and, as a rule, is measured in KJ / mol or kcal / mol.
If the reaction occurs with heat release (q\u003e 0), it is called exothermic, and if with heat absorption (q< 0) – эндотермической.
If it is schematically to portray the energy profile of the reaction, then for endothermic reactions, products are above energy than reagents, and for exothermic - on the contrary, the reaction products are located below energy (more stable) than reagents.
It is clear that the larger the substance reacts, the greater the amount of energy is separated (or absorbed), i.e. The thermal effect is directly proportional to the amount of substance. Therefore, the attitude of the thermal effect to 1 mol of the substance is due to our desire to compare the thermal effects of various reactions.
Lecture 6. Thermochemistry. The thermal effect of the chemical reaction Example 1. With the restoration of 8.0 g of copper (II) oxide of hydrogen, metal copper and water pairs were formed and 7.9 kJ heat out. Calculate the thermal effect of the reaction of the reduction of copper oxide (II).
Decision . Cuo reaction equation (TV.) + H2 (G.) \u003d Cu (TV.) + H2 O (G.) + Q (*)
Make a proportion during the restoration of 0.1 mol - 7.9 kJ is allocated during restoration of 1 mol - x kj
Where x \u003d + 79 kj / mole. Equation (*) takes
Cuo (TV.) + H2 (G.) \u003d Cu (TV.) + H2 O (G.) +79 kJ
Thermochemical equation- This is a chemical reaction equation, which indicates the aggregate state of the components of the reaction mixture (reagents and products) and the thermal effect of the reaction.
So as to melt the ice or evaporate water, it is necessary to spend certain amounts of heat, while during freezing of liquid water or condensation of the water vapor the same amounts are allocated. That is why it is cold when we come out of the water (evaporation of water from the body surface requires energy costs), and the sweating is a biological protective mechanism from the overheating of the body. On the contrary, the freezer freezes water and heats the surrounding room, giving it an excessive heat.
This example shows the thermal effects of changes in the aggregate state of water. The heat of melting (at 0o C) λ \u003d 3.34 × 105 J / kg (physics), or QL. \u003d - 6.02 kJ / mol (chemistry), heat evaporation (vaporization) (at 100o c) q \u003d 2.26 × 106 J / kg (physics) or Qisp. \u003d - 40.68 kJ / mol (chemistry).
melting
evaporation |
|||||
obr, 298.
Lecture 6. Thermochemistry. The thermal effect of the chemical reaction is of course, sublimation processes are possible when the solid
it goes into the gas phase, bypassing the liquid state and the inverse processes of deposition (crystallization) from the gas phase, it is also possible to calculate or measure the thermal effect.
It is clear that in each substance there are chemical bonds, therefore, each substance has some energy reserve. However, not all substances can be turned into each other with one chemical reaction. Therefore, agreed to introduce a standard state.
Standard state of substance- This is an aggregate state of a substance at a temperature of 298 K, a pressure of 1 atmosphere in the most stable allotropic modication in these conditions.
Standard conditions- This is the temperature of 298 K and pressure 1 atmosphere. Standard conditions (standard status) is indicated by the index0.
Standard Heat Formation Connection it is called the thermal effect of the chemical reaction of the formation of this compound from simple substances taken in their standard state. The heat of the compound formation is indicated by the Q symbol.0 For a variety of compounds, standard thermal education is given in the reference books of physicochemical values.
The standard heat of the formation of simple substances is equal to 0. For example, Q0 arr., 298 (O2, gas) \u003d \u200b\u200b0, q0 arr., 298 (C, TV, graphite) \u003d 0.
For example . Record the thermochemical equation for the formation of copper sulfate (II). From the reference book Q0 arr, 298 (CUSO4) \u003d 770 kJ / mol.
Cu (TV.) + S (TV.) + 2O2 (G.) \u003d Cuso4 (TV) + 770 kJ.
Note: The thermochemical equation can be recorded for any substance, however, it is necessary to understand that in real life the reaction occurs in a completely different way: from the listed reagents are formed when heated oxide of copper (II) and sulfur (IV), but copper sulfate (II) is not formed . Important conclusion: The thermochemical equation is a model that allows calculations, it is well consistent with other thermochemical data, but does not withstand verification of practice (that is, unable to correctly predict the possibility or inability to reaction).
(B j) - Σ a i × q arr 0, 298 i
Lecture 6. Thermochemistry. Thermal effect of chemical reaction
Clarification. In order not to mislead you, I will immediately add that chemical thermodynamics may predict the possibility / impossibility of reactionHowever, this requires more serious "tools" that go beyond the school course of chemistry. The thermochemical equation compared to these techniques is the first step on the background of the Heopse pyramid - without it can not do, but not high.
Example 2. Calculate the thermal effect of water condensation weighing 5,8g. The condensation process is described by the thermochemical equation H2 O (G.) \u003d H2 O (g.) + Q - condensation usually exothermic heat condensation process at 25o C 37 kJ / mol (directory).
Consequently, Q \u003d 37 × 0.32 \u003d 11.84 kJ.
In the 19th century, the Russian chemist, who studied the thermal effects of reactions, was experimentally established the law of energy conservation in relation to chemical reactions - the law of the hess.
The thermal effect of the chemical reaction does not depend on the process path and is determined only by the difference in finite and initial states.
From the point of view of chemistry and mathematics, this law means that we are free to calculate the process to choose any "trajectory of calculation", because the result does not depend on it. For this reason, the very important law of hess has an incredibly important corollary of the GESS law.
The thermal effect of the chemical reaction is equal to the sum of the heat of the formation of the reaction products net of the heat of the formation of reagents (taking into account the stoichiometric coefficients).
From the point of view of common sense, this investigation corresponds to the process in which all the reagents first turned into simple substances, which were then gathered in a new way, so that the reaction products were obtained.
In the form of the equation, the consequence of the GESS law looks like the reaction equation: A 1 A 1 + A 2 A 2 + ... + A n A N \u003d B 1 B 1 + B 2 B 2 + ... B
At the same time, A I I IB J are stoichiometric coefficients, A I - reagents, B j - reaction products.
Then the consequence of the GESSA law has the form q \u003d σ b j × q arr. 0, 298
k bk + q
(A i)
Lecture 6. Thermochemistry. The thermal effect of a chemical reaction Since the standard heat of the formation of many substances
a) Committed to special tables or b) can be determined experimentally, it becomes possible to predict (calculate) the thermal effect of a very large number of reactions with sufficiently high accuracy.
Example 3. (The consequence of the GESS law). Calculate the thermal effect of the steam conversion of methane occurring in the gas phase under standard conditions:
CH4 (G.) + H2 O (G.) \u003d CO (G.) + 3 H2 ()
Determine whether this reaction is exothermic or endothermic?
Solution: The consequence of the GESS law
Q \u003d 3 Q0 | D) + Q 0 | (CO, D) -Q 0 | D) -q 0 | O, d) - in general. |
|||||
obr, 298. | obr, 298. | obr, 298. | obr, 298. | ||||||
Q Ob0. | 298 (H 2, D) \u003d 0 | Simple substance in standard condition |
|||||||
From the reference book we find the warmth of the formation of the remaining components of the mixture.
O, d) \u003d 241.8 | (Co, d) \u003d 110.5 | D) \u003d 74.6 | |||||||||
obr, 298. | obr, 298. | obr, 298. | |||||||||
We substitute the values \u200b\u200bto the equation
Q \u003d 0 + 110.5 - 74.6 - 241.8 \u003d -205.9 kJ / mol, the reaction is highly endothermich.
Answer: Q \u003d -205.9 kJ / mol, endothermal
Example 4. (Application of the GESSA). The warmth of reactions are known
C (TV.) + ½ O (g.) \u003d Co (g.) + 110.5 kJ
C (TV.) + O2 (G.) \u003d CO2 (G.) + 393.5 kJ Find the thermal effect of reaction 2CO (G.) + O2 (G.) \u003d 2CO2 (g.). Decision multiplying the first and second equation on 2
2c (TV.) + O2 (g.) \u003d 2Co (g.) + 221 kJ 2c (TV.) + 2O2 (G.) \u003d 2CO2 (G.) + 787 kJ
Submount from the second equation the first
O2 (G.) \u003d 2CO2 (G.) + 787 KJ - 2CO (G.) - 221 kJ,
2CO (G.) + O2 (G.) \u003d 2CO2 (G.) + 566 kJ Answer: 566 kJ / mol.
Note: When studying thermochemistry, we consider the chemical reaction from the outside (outside). On the contrary, chemical thermodynamics - the science of behavior of chemical systems - considers the system from the inside and operates with the concept of "enthalpy" H as the thermal energy of the system. Entalpy, such
Lecture 6. Thermochemistry. The thermal effect of the chemical reaction is the same meaning as the amount of heat, but has the opposite sign: if the energy is distinguished from the system, its environment gets and heated, and the system loses energy.
Literature:
1. Tutorial, V.V. Eremin, N.E. Kuzmenko et al., Chemistry grade 9, paragraph 19,
2. Educational and methodological manual "Fundamentals of general chemistry" Part 1.
Compilers - S.G. Baram, I.N. Mironova. - take with you! For the next seminar occupation
3. A.V. Manuilles. Basics of chemistry. http://hemi.nsu.ru/index.htm.
§9.1 The thermal effect of the chemical reaction. The main laws of thermochemistry.
§9.2 ** Thermochemistry (continued). The heat of the formation of the substance from the elements.
Standard enthalpy education.
Attention!
We go to solve the settlement tasks, therefore, the calculator is also desirable for seminars in chemistry.
Thermochemistry studies the thermal effects of chemical reactions. In many cases, these reactions occur at a constant volume or constant pressure. From the first law of thermodynamics, it follows that under these conditions of heat is a function function. With a constant volume of heat equal to the change in internal energy:
and at constant pressure, the change in enthalpy:
These equalities in the application to chemical reactions are essential gESSA law:
The thermal effect of the chemical reaction flowing at constant pressure or constant volume does not depend on the reaction path, and is determined only by the state of reagents and reaction products.
In other words, the thermal effect of the chemical reaction is to change the status function.
In thermochemistry, in contrast to other thermodynamic applications, the heat is considered positive if it is highlighted in the environment, i.e. if a H. < 0 или U.
< 0. Под тепловым эффектом химической реакции
понимают значение H. (which is called simply "enthalpy reaction") or U. reactions.
If the reaction proceeds in solution or solid phase, where the change in the volume is slightly,
H. = U. + (pV) U.. (3.3)
If perfect gases are involved in the reaction, then at a constant temperature
H. = U. + (pV) = U. + n. Rt., (3.4)
where n is a change in the number of moles of gases in the reaction.
In order to facilitate the comparison of the enthalpium of various reactions, use the concept of "standard state". Standard condition is the state of pure substance at a pressure of 1 bar (\u003d 10 5 pa) and a given temperature. For gases, this is a hypothetical condition at a pressure of 1 bar, which has the properties of infinitely sparse gas. The enthalpy of the reaction between substances in standard conditions at temperatures T., denote ( r. Means "REACTION"). In thermochemical equations, not only formulas of substances, but also their aggregate states or crystalline modifications are indicated.
From the law of the hess, important consequences flow, which allow to calculate the enthalpy of chemical reactions.
Corollary 1.
![]()
equal to the difference in standard enthalpy formation of reaction products and reagents (taking into account stoichiometric coefficients):
Standard enthalpy (heat) of substance formation (f. means "formation") at a given temperature called the enthalpy of the formation of the formation of one praying of this substance from elementslocated in the most sustainable standard state. According to this definition, the enthalpy of formation of the most stable simple substances in the standard state is 0 at any temperature. Standard enthalpies of formation of substances at a temperature of 298 K are given in reference books.
The concepts of "enthalpy of education" are used not only for conventional substances, but also for ions in solution. At the same time, the H + ion is received per point of reference, for which the standard enthalpy of formation in aqueous solution is supposed to be zero: ![]()
Corollary 2. Standard enthalpy of chemical reaction
![]()
equal to the difference of enthalpy of combustion of reagents and reaction products (taking into account stoichiometric coefficients):
(c. Means "Combucer"). The standard enthalpy (heat) of the combustion of the substance is called the enthalpy of the reaction of the total oxidation of one praying substance. This consequence is usually used to calculate the thermal effects of organic reactions.
Corollary 3. The enthalpy of the chemical reaction is equal to the difference of the energy of the torn and the resulting chemical bonds.
Communication energy A- B is called the energy necessary to break the bond and dilution of the resulting particles to the infinite distance:
AB (g) A (g) + B (g).
Communication energy is always positive.
Most thermochemical data in reference books are given at a temperature of 298 K. to calculate thermal effects at other temperatures use kirchhoff equation:
(Differential form) (3.7)
 (integral form) (3.8)
(integral form) (3.8)
where C P. - The difference of the isobaric heat-capacity of the reaction products and the starting materials. If difference T. 2 - T. 1 is small, then you can take C P. \u003d const. With a large temperature difference, it is necessary to use the temperature dependence C P.(T.) Type:
where coefficients are a., b., c. etc. For individual substances, they take from the directory, and the sign indicates the difference between the products and reagents (taking into account the coefficients).
Examples
Example 3-1. Standard enthalpies of formation of liquid and gaseous water at 298 K are equal to -285.8 and -241.8 kJ / mol, respectively. Calculate the enthalpy of water evaporation at this temperature.
Decision. Education enthalpy correspond to the following reactions:
H 2 (g) + ѕO 2 (g) \u003d H 2 O (g), H. 1 0 = -285.8;
H 2 (g) + ѕO 2 (g) \u003d H 2 O (g), H. 2 0 = -241.8.
The second reaction can be carried out in two stages: first burn hydrogen to form liquid water by the first reaction, and then evaporate the water:
H 2 O (g) \u003d H 2 O (g), H. 0 is \u003d?
Then, according to the law of the hess,
H. 1 0 + H. 0 is \u003d. H. 2 0 ,
from H. 0 isp \u003d -241.8 - (-285.8) \u003d 44.0 kJ / mol.
Answer. 44.0 kJ / mol.
Example 3-2. Calculate the enthalpy reaction
6C (g) + 6h (g) \u003d C 6 H 6 (g)
a) on the enthalpies of education; b) on communication energies, under the assumption that double bonds in the molecule C 6 H 6 are fixed.
Decision. a) Education enthalpy (in KJ / mole) we find in the directory (for example, p.w.atkins, Physical Chemistry, 5th Edition, PP. C9-C15): F H. 0 (C 6 H 6 (g)) \u003d 82.93, F H. 0 (C (g)) \u003d 716.68, F H. 0 (H (d)) \u003d 217.97. The enthalpy reaction is:
R H. 0 \u003d 82.93 - 6 716.68 - 6 217.97 \u003d -5525 kJ / mol.
b) In this reaction, chemical bonds are not broken, but only formed. In the approximation of fixed double bonds, the C 6 H 6 molecule contains 6 bonds C-H, 3 of communication C - C and 3 of communication C \u003d c. Energy links (in KJ / mol) (p.w.atkins, Physical Chemistry, 5th Edition, p. C7): E.(C- H) \u003d 412, E.(C- C) \u003d 348, E.(C \u003d C) \u003d 612. Reaction enthalpy is equal to:
R H. 0 \u003d - (6 412 + 3 348 + 3 612) \u003d -5352 kJ / mol.
The difference with the exact result is -5525 kJ / mol is due to the fact that in the benzene molecule there are no single bonds of C - C and double bonds C \u003d C, and there are 6 aromatic bonds with C C.
Answer. a) -5525 kj / mol; b) -5352 kj / mole.
Example 3-3. Using reference data, calculate the enthalpy of the reaction
3CU (TV) + 8HNO 3 (AQ) \u003d 3CU (NO 3) 2 (AQ) + 2NO (g) + 4H 2 O (g)
Decision. The abbreviated ionic reaction equation has the form:
3CU (TV) + 8H + (AQ) + 2NO 3 - (AQ) \u003d 3CU 2+ (AQ) + 2NO (g) + 4H 2 O (g).
According to the GESS law, the enthalpy reaction is equal to:
R H. 0 = 4 F H. 0 (H 2 O (g)) + 2 F H. 0 (NO (g)) + 3 F H. 0 (Cu 2+ (AQ)) - 2 F H. 0 (NO 3 - (AQ))
(Enhaulpia of the formation of copper and ion H + are equal, by definition, 0). Subthantizing Education Education (p.w.atkins, Physical Chemistry, 5th Edition, PP. C9-C15), we find:
R H. 0 \u003d 4 (-285.8) + 2 90.25 + 3 64.77 - 2 (-205.0) \u003d -358.4 KJ
(per three praying copper).
Answer. -358.4 KJ.
Example 3-4. Calculate the enthalpy of the combustion of methane at 1000 K if the enthalpy of formation is given at 298 to: F H. 0 (CH 4) \u003d -17.9 kcal / mol F H. 0 (CO 2) \u003d -94.1 kcal / mol F H. 0 (H 2 O (g)) \u003d -57.8 kcal / mol. The heat capacity of the gases (in Cal / (moth. K)) in the range from 298 to 1000 K is equal:
C P (CH 4) \u003d 3.422 + 0.0178. T., C P.(O 2) \u003d 6.095 + 0.0033. T.,
C P (CO 2) \u003d 6.396 + 0.0102. T., C P.(H 2 O (d)) \u003d 7.188 + 0.0024. T..
Decision. Enhaulpia Methane Combustion Reaction
CH 4 (g) + 2O 2 (g) \u003d CO 2 (g) + 2H 2 O (g)
at 298 to equal:
94.1 + 2 (-57.8) - (-17.9) \u003d -191.8 kcal / mol.
Find the difference of heat-capacity as a function of temperature:
C P. = C P.(CO 2) + 2 C P.(H 2 O (d)) - C P.(CH 4) - 2 C P.(O 2) \u003d
= 5.16 - 0.0094T. (Cal / (moth. K)).
The enthalpy of the reaction at 1000 to calculation according to the Kirchhoff equation:
= +  = -191800 + 5.16
= -191800 + 5.16
(1000-298) - 0.0094 (1000 2 -298 2) / 2 \u003d -192500 Cal / mol.
Answer. -192.5 kcal / mol.
TASKS
3-1. How much heat will be required for the translation of 500 g Al (T.pl. 658 o C, H. 0 pl \u003d 92.4 cal / g), taken at room temperature, in the molten state, if C P.(Al TV) \u003d 0.183 + 1.096 10 -4 T. Cal / (g k)?
3-2. Standard enthalpy reaction CACO 3 (TV) \u003d Cao (TV) + CO 2 (g) occurring in an open vessel at a temperature of 1000 K, equal to 169 kJ / mol. What is equal to the heat of this reaction flowing at the same temperature, but in the closed vessel?
3-3. Calculate the standard internal energy of the formation of liquid benzene at 298 K, if the standard enthalpy of its formation is 49.0 kJ / mol.
3-4. Calculate the enthalpy of formation N 2 O 5 (d) at T. \u003d 298 K based on the following data:
2no (g) + o 2 (g) \u003d 2NO 2 (g), H. 1 0 \u003d -114.2 kJ / mol,
4NO 2 (g) + o 2 (g) \u003d 2n 2 O 5 (g), H. 2 0 \u003d -110.2 kJ / mol,
N 2 (g) + o 2 (g) \u003d 2no (g), H. 3 0 \u003d 182.6 kJ / mol.
3-5. Enhaulpia combustion-glucose, -fructosis and sucrose at 25 ° C are equal to -2802,
-2810 and -5644 kJ / mol, respectively. Calculate the warmth of the hydrolysis of sucrose.
3-6. Determine the enthalpy of formation of the formation of B 2 H 6 (g) when T. \u003d 298 to from the following data:
B 2 H 6 (g) + 3O 2 (g) \u003d b 2 O 3 (TV) + 3H 2 O (g), H. 1 0 \u003d -2035.6 kJ / mol
2B (TV) + 3/2 O 2 (g) \u003d B 2 O 3 (TV), H. 2 0 \u003d -1273.5 KJ / Mol,
H 2 (g) + 1/2 O 2 (g) \u003d H 2 O (g), H. 3 0 \u003d -2241.8 KJ / mol.
3-7. Calculate the heat of the formation of zinc sulfate from simple substances when T. \u003d 298 K based on the following data.