Of the characteristics of the elements atoms listed below. Chemistry
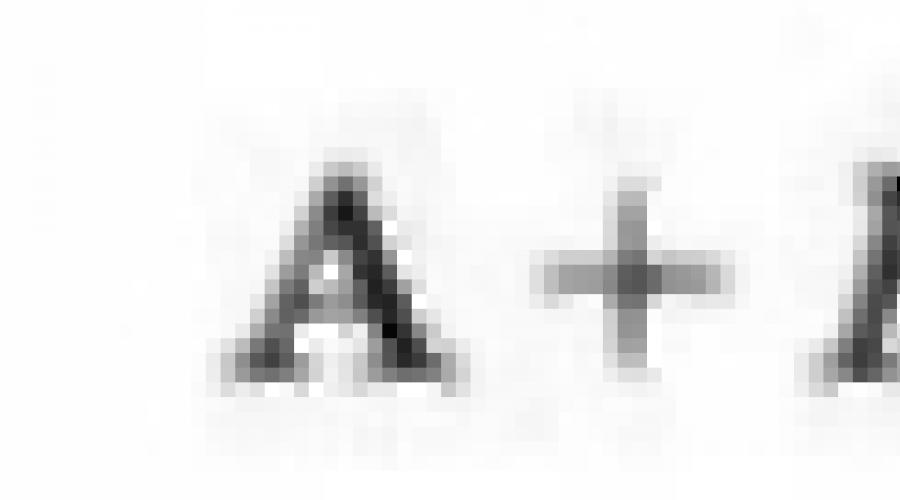
Read also
Examination No. 2 contains tasks to the following topics:
- Periodic system
- The frequency of changing the properties of the elements and their compounds.
- Chemical bond. Method Sun.
- Chemical bond. MO method.
- Chemical bond. Ion connection.
- Chemical bond in complex compounds.
Test for knowledge check:
1. Of the characteristics of the elements atoms listed below periodically change
(1) atom nuclei charge;
(2) relative atomic mass;
(3) the number of energy levels in the atom;
(4) The number of electrons in the external energy level.
2. Inside the period, an increase in the sequence number of the element is usually accompanied by
(1) with a decrease in the atomic radius and an increase in the electronegativity of the atom;
(2) an increase in the atomic radius and a decrease in the electronegability of the atom;
(3) with a decrease in the atomic radius and decrease in the electronegability of the atom;
(4) an increase in the atomic radius and an increase in the electronegability of the atom.
3. Atom of which element is the easiest way to give one electron (numbers mean the sequence number of the element):
(1) sodium, 11; (2) magnesium, 12; (3) aluminum, 13; (4) silicon, 14?
4. Atoms of elements 1A of the periodic system of elements have the same number
(1) electrons at the external electronic level;
(2) neutrons;
(3) all electrons.
5. Elements are located in the order of increasing electronegability in the row
(1) AS, SE, CL, F; (2) C, I, B, SI; (3) br, p, h, sb; (4) O, SE, BR, TE.
6. In the second and third periods of the periodic system as the size of the elements atoms decreases
(1) the size of their ions is also reduced;
(2) Electricity decreases;
(3) Metal properties of elements weaken;
(4) Metal properties of elements are enhanced.
7. Which number includes only transitional elements:
(1) elements 11, 14, 22, 42; (2) elements 13, 33, 54, 83;
(3) elements 24, 39, 74, 80; (4) Elements 19, 32, 51, 101?
8. Which of the elements below has chemical properties, allowing to talk about its similarity with the calcium element:
(1) Carbon. FROM; (2) sodium, Na; (3) Potassium. TO; (4) Strontium, SR?
9. The non-metallic properties of the elements located in the main subgroups of the Periodic System D. I. Mendeleev are most pronounced in those of those who are located
(1) at the top of the subgroup;
(2) at the bottom of the subgroup;
(3) in the middle of the subgroup;
(4) In all elements, the subgroup is expressed in about the same degree.
10. Which number of elements is represented in ascending order of atomic radius:
(1) O, S, SE, those; (2) C, N, O, F; (3) Na, Mg, Al, Si; (4) I, BR, CL, F?
11. Metal nature of the properties of elements in a series of MG-CA-SR-BA
(1) decreases;
(2) increases;
(3) does not change;
12. Non-metallic nature of the properties of elements in a number of N-P-AS-SB-BI
(1) decreases;
(2) increases;
(3) does not change;
(4) decreases, and then increases.
13. Which steam in the specified set of elements - CA, P, SI, AG, NI, AS - has the most similar chemical properties:
(1) SA, Si; (2) AG, Ni; (3) P, AS; (4) Ni, r?
14. According to its chemical properties, radioactive element of radium is closest to
(1) cesium; (2) Barium; (3) Lanthan; (4) Actinia.
15. Based on the position of the Lanthan element in the periodic system, it is safe to assert that for lanthanides, the most characteristic degree of oxidation will be
(1) +1; (2) +2; (3) +3; (4) +4.
16. Main properties of hydroxides of elements of the group 1a as the sequence number increases
(1) decrease;
(2) increase;
(3) remain unchanged;
(4) decrease, and then increase.
17. Based on the position of elements in the periodic system, the most likely joint of Germany with selenium can be depicted by the formula.
18. The hypothetical element Z forms ZCL 5 chloride. What is the most likely formula for its oxide:
(1) ZO 2; (2) ZO 5; (3) Z 2 O 5; (4) z 5 o 2?
19. Simple substances of which elements have the greatest similarity of physical and chemical properties:
(1) Li, S; (2) BE, CL; (3) f, cl; (4) Li, F?
20. Of the elements of the third period below, the most pronounced non-metallic properties has
(1) aluminum; (2) silicon; (3) sulfur; (4) Chlorine.
21. Of the above elements IIIA, group pronounced non-metallic properties has
(1) Bor; (2) aluminum; (3) Gallium; (4) India.
22. Which of the above elements of the fourth period of the periodic system manifests the same valence values \u200b\u200bin their hydrogen compound and in the highest oxide:
(1) bromine; (2) Germany; (3) arsenic; (4) Selenium?
23. The nature of oxides in the row P 2 O 5 -Sio 2 -L 2 o z -mgo is changed as follows:
(1) from the main to acidic;
(2) from the acid to the main;
(3) from the main to amphoter;
(4) from amphoteric to acidic.
24. Write the formulas of higher oxides of elements and corresponding acids; Name these acids:
25. Based on the position of the element in the periodic system, write its compounds that are listed below:
26. From the above list of elements: BE, B, C, N, Al, Si, P, S, GA, GE, AS, BR - EO 2 type oxides form, and the hydrides of the type AN 4 -.
27. Based on the position of the element in the periodic system, output the formulas for its highest oxide and hydroxide and indicate their nature:
28. The element with the sequence number 34 forms a hydrogen compound, higher oxide and hydroxide. The latter is manifested
(1) acid properties;
(2) basic properties;
(3) amphoteric properties.
29. The maximum number of chemical elements that can fill in the sixth period of the periodic system should be equal to
(1) 8; (2) 18; (3) 32; (4) 50.
30. The maximum number of items in the seventhomeriod should be
(1) 18; (2) 32; (3) 50; (4) 72.
31. In the seventh period, the latter must be element by the sequence number
(1) 118; (2) 114; (3) 112; (4) 110.
32. The properties of alkali metals should be expected from elements with sequence numbers
(1) 111 and 190; (2) 119 and 169; (3) 137 and 187; (4) 155 and 211.
33. The configuration of the bismuth orbitals of valence electrons coincides with
(1) selenium and tellurium;
(2) nitrogen and phosphorus;
(3) silicon and germanium;
(4) Niobium and Tanthal.
34. The element with the sequence number 117 should be attributed to
(1) alkaline metal lamas; (3) halogens;
(2) alkaline earth metals; (4) transitional elements.
35. The maximum valence of lead in oxygen compounds is:
(1) II; (2) IV; (3) VI; (4) VIII.
36. The type of orbitals of valence electrons in India coincides with
(1) AM and FR; (2) PB and SN; (3) Al and Ga; (4) CU and AG.
37. Titan refers to
(1) s.-; (2) p.-; (3) d.-; (4) f.- elements.
38. Maximum valence of bromine in oxygen compounds
(1) i; (2) iii; (3) v; (4) VII.
39. The seventh period of the system of elements must end the element with the sequence number
(1) 108; (2) 110; (3) 118; (4) 128.
40. The angle between the links of H-e is the largest in the molecule of the connection
(1) H 2, (2) H 2 SE; (3) H 2 S; (4) H 2 O.
41. In a row of K-CA-SC-TI, the radius of atoms (decreases, increases).
42. Energy that is specified in the SL ° (G.) → CL + equation (G.) + E - 1254 kJ, is for chlorine atom
(1) chemical bond energy;
(2) ionization energy;
(3) electronegitability;
(4) affinity to the electron.
43. The affinity for the electron is called
(1) the energy required for the separation of an electron from an unexcited atom;
(2) the ability of the atom of this item to delay the electron density;
(3) Electron transition to a higher energy level;
(4) Energy isolating when an electron is attached to an atom or ion.
44. Which of the elements is the greatest value of the ionization energy:
(1) Li; (2) f; (3) Fe; (4) I?
45. Energy spent on removing one electron from an element atom in a gaseous state, in magnesium
(1) less than sodium, and more than aluminum;
(2) more than sodium, and less than that of aluminum;
(3) less than sodium and aluminum;
(4) more than sodium and aluminum.
46. \u200b\u200bBased on the analysis of the electronic structures of atoms and positions of elements in the periodic system, specify which of each two atoms below has greater affinity to the electron:
(1) Potassium or calcium;
(2) sulfur or chlorine;
(3) hydrogen or lithium?
47. Chemical elements are located in order of increasing electronegability in the row
(1) Si, P, SE, RR, SL, O; (2) Si, P, R, SE, C1, O;
(3) p, si, fra, se, c1, o; (4) SE, Si, P, R, C1, O.
48. What a number of elements is located as they increase their atomic radii:
(1) Na, Mg, Al, Si; (3) O, S, SE, those;
(2) C, O, N, F; (4) I, BR, C1, F?
49. In a number of alkali metal (from Li to Cs), cesium is the least electronegative. This is explained by what he has
(1) the largest number of neutrons in the kernel;
(2) a greater number of valence electrons compared to other elements;
(3) a large atomic mass;
(4) Valented electrons, to the greatest degree removed from the nucleus of the atom.
50. Isoelectronic called ions having the same number of electrons and the same structure of the external electron level. Ions about 2-, F -, Na +, Mg 2+, A1 3+ have an electronic configuration of the noon's noo gas and are arranged in ascending order of atomic masses of the elements. At the same time their ion radii
(1) practically do not change;
(2) decrease;
(3) increase;
(4) decrease, then increase.
51. An example of a non-polar molecule having a polar covalent bond will be
(1) N 2; (2) H 2 O; (3) NH 3; (4) CCL 4.
52. Of the above molecules: H 2, O 2, H 2 O, CO 2, CH 4, H 2 S - polar are.
53. In which of the compounds between atoms, a covalent bond on the donor-acceptor mechanism is formed:
(1) KSL; (2) NH 4 Cl; (3) CCL 4; (4) CO 2?
54. Valental orbitals of beryllium atom in the hydride molecule of beryllium hybridized by type
(1) sp.; (2) sP 2.; (3) sP 3.;(4) d 2 SP 3,
and the molecule has a structure:
55. Valental orbitals of the boron atom in the TF 3 molecule are hybridized by type
(1) sp.; (2) sP 2.; (3) sP 3.;(4) d 2 SP 3,
and the molecule has a structure:
(a) linear; (c) tetrahedral;
(b) flat; (d) octahedral.
56. The presence of four equivalent bonds of the C - H in the methane molecule is explained by the fact that
(1) Mutual repulsion of four electronic pairs occurs;
(2) carbon atom hybridized to form four sP 3.orbitals;
(3) carbon atom has one s.- and three r- valence electron;
(4) carbon atom has two s- and two r- valence electron.
Answers:
1. (4) The number of electrons in the external energy level.
2. (1) with a decrease in the atomic radius and an increase in the electronegativity of the atom.
3. (1) Sodium, 11.
4. (1) electrons at the external electronic level.
5. (1) AS, SE, Cl, F.
6. (3) Metal properties of elements weaken.
7. (3) Elements 24, 39, 74, 80.
8. (4) Strontsia, Sr.
9. (1) At the top of the subgroup.
10. (1) O, S, SE, those.
11. (2) increases.
12. (1) decreases.
14. (2) Barium.
16. (2) increase.
18. (3) Z 2 O 5.
20. (4) Chlorine.
22. (2) Germany.
23. (2) From the acid to the main one.
26. EO 2 type oxides form C, Si, GE, and the hydrides of the type An 4 - C, Si, Ge.
28. H 2 S, SEO 3 and H 2 SEO 4. (1) Acid Properties.
32. (2) 119 and 169.
33. (2) Nitrogen and phosphorus.
34. (3) halogens.
36. (3) Al and Ga.
37. (3) d.- elements.
41. decreases.
42. (2) ionization energy.
43. (4) Energy isolating when an electron is connected to an atom or ion.
45. (4) more than sodium and aluminum.
46. \u200b\u200b(1) Potassium; (2) chlorine; (3) hydrogen.
47. (1) Si, P, SE, WR, SL, O.
48. (3) O, S, SE, those.
49. (4) Valence electrons, to the greatest degree removed from the nucleus of the atom.
50. (2) decrease.
52. H 2 O, H 2 S.
53. (2) NH 4 Cl.
54. (1) sp., (a) linear.
55. (2) sP 2., (b) flat.
56. (2) carbon atom is hybridized with the formation of four sP 3.orbitals.
Tasks for individual estimated graphics work:
For an element with a sequence number equal to the number of options to carry out the following calculations:
1. Write an electronic formula of the element and show graphically filling with electrons of all atomic orbitals.
3. Determine the mass of one element atom and its volume.
4. Determine the mass of one molecule of a simple substance of the element.
5. Based on the position of the element in the PS, lists the possible degrees of oxidation of the atom of the element in connections with other elements.
6. Write an oxide formula, chloride, hydride, sulfide.
8. Calculate the length of the dipole of the hydrogen and oxygen connection of the element.
9. Pail into touch in the molecule of a simple substance of the element using the Sun method.
10. Pail into the molecule of the element simple substance using the energy diagram of the MO method, specify the multiplicity of communication and write a formula.
11. Specify the type of hybridization of an atom of an element in molecules of all possible oxides (in the case of oxygen - molecules of hydrogen connections).
12. Specify all types of bonds (σ, π, δ) in oxide molecules (in the case of oxygen - hydrogen compounds).
13. Specify the values \u200b\u200bof valence angles in oxide molecules (in the case of oxygen - hydrogen compounds molecules).
14. Specify the form of oxide molecules (in the case of oxygen - hydrogen compounds molecules).
15. Calculate the energy of the ionic compound of AV and the energy of the interaction of ions A + and B.
For options 1, 5, 6, 7, 8, 9, 14, 15, 16, 17: a - potassium, B is an element with a sequence number equal to the element number.
For options 3, 4, 11, 12, 13, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28: B - chlorine, an element with a sequence number equal to the element number.
For options 2, 10, 18: a - an element with a sequence number equal to (version of +1), B is an element with a sequence element equal to (option -1).
Literature.
1. Kulman A.G. Collection of tasks for general chemistry, ed. 2nd, recreation., And add. - M.: Higher. shk. 1975.
2. Maslov E.I. , Holboray Z.E. Collection of tasks and exercises in chemistry, 5th ed., Pererab. and add-m.: Higher. shk. 1997.
The atomic number of the element shows:
a) the number of elementary particles in the atom; b) the number of nucleons in the atom;
c) the number of neutrons in the atom; d) the number of protons in the atom.
The most correct is the statement that chemical elements in PSE are arranged in ascending order:
a) the absolute mass of their atoms; b) relative atomic mass;
c) the number of nucleons in atomic nuclei; d) the charge of the atomic nucleus.
The frequency in changing the properties of chemical elements is the result:
a) increase the number of electrons in atoms;
b) increasing the charges of atomic nuclei;
c) increase atomic mass;
d) periodicity in the change in the electronic structures of atoms.
Of the characteristics listed below, the element atoms are periodically changed as the sequence number of the element is grown:
a) the number of energy levels in the atom;
b) relative atomic mass;
c) the number of electrons in the external energy level;
d) the charge of the atom nucleus.
Select pairs in which each characteristic of the atom varies periodically with an increase in the proton number of the element:
a) the energy of ionization and energy of an electron affinity;
b) radius and mass;
c) electronegativity and total electrons;
d) metal properties and the number of valence electrons.
Select Proper Approval for ElementsV.A group:
a) all atoms have the same number of electrons;
b) all atoms have the same radius;
c) all atoms have the same number of electrons on the outer layer;
d) All atoms have a maximum valence equal to the group number.
Some element has the following electrons configuration:nS. 2 (n.-1) d. 10 nP. 4 . In which group of the periodic system is this element?
a) IVT group; b) VIV group; c) IVA Group; d) VIA group.
In PSE periods with increasing charges of atomic nucleinot Changes: a) the mass of atoms; b) the number of electronic layers; c) the number of electrons on the outer electronic layer; d) Radius of atoms. |
|
In what row chemical elements are located in order of increasing their atomic radius? a) Li, BE, B, C; b) BE, MG, CA, SR; c) n, o, f, ne; d) Na, Mg, Al, Si. |
|
The lowest ionization energy among stable atoms has: a) lithium; b) barry; c) cesium; d) sodium. |
|
Electricity of the elements increases in a row: a) p, si, s, o; b) cl, f, s, o; c) TE, SE, S, O; d) O, S, SE, TE. |
|
In a row of elementsNa. MG. Al SI P. S. Cl. from left to right: a) increases electronegacity; b) the energy of ionization decreases; c) the number of valence electrons increases; d) Metal properties decrease. |
|
Specify the most active metal of the fourth period: a) calcium; b) potassium; c) chrome; d) zinc. |
|
Specify the most active metal IIA group: a) beryllium; b) barry; c) magnesium; d) calcium. |
|
Specify the most active nonmetall VIIA group: a) iodine; b) bromine; c) fluorine; d) chlorine. |
|
Choose the right statements: a) In IA-VIII, only elements S- and b) in IV-VIIIV groups there are only D-elements; c) all D-elements are metals; d) the total number of s elements in PSE is 13. |
|
With an increase in the atomic number of the element in the VA group increase: a) metal properties; b) the number of energy levels; c) the total number of electrons; d) the number of valence electrons. |
|
The p-elements refers: a) potassium; b) sodium; c) magnesium; d) arsenic. |
|
Which family of elements is aluminum? a) s-elements; b) p-elements; c) d-elements; d) F-elements. |
|
Specify a series in which there are onlyd.- elements: a) Al, SE, LA; b) Ti, GE, SN; c) Ti, V, CR; d) LA, CE, HF. |
What row shows the characters of elements s, p and d-families? a) h, he, li; b) h, ba, al; c) be, c, f; d) mg, p, cu. |
|
Atom of which element of the IV period contains the largest number of electrons? a) zinc; b) chrome; c) bromine; d) Krypton. |
|
At the atom of which element, the electrons of the external energy level are most firmly connected to the nucleus? a) potassium; b) carbon; c) fluorine; d) france. |
|
The force of attraction of valence electrons to the nucleus of the atom decreases into a number of elements: a) na, mg, al, si; b) rb, k, na, li; c) SR, CA, MG, BE; d) li, na, k, rb. |
|
The element with the sequence number 31 is: a) in the group III; b) small period; c) the big period; d) in group A. |
|
From the following electronic formulas, select those that match the P-elementsV. Period: a) 1s 2 2s 2 2p 6 3S 2 3p 6 3D 1 4S 2 4P 6 4D 1 5S 2 5P 1; b) 1s 2 2S 2 2P 6 3S 2 3P 6 3D 1 4S 2 4P 6 5S 2; c) 1S 2 2S 2 2P 6 3S 2 3P 6 3D 1 4S 2 4P 2; d) 1S 2 2S 2 2P 6 3S 2 3P 6 3D 1 4S 2 4P 6 4D 1 5S 2 5P 6. |
|
From the above electronic formulas, select those that correspond to the chemical elements forming the highest oxide of the composition 2 ABOUT 3 : a) 1s 2 2S 2 2p 6 3S 2 3P 1; b) 1S 2 2S 2 2P 6 3S 2 3P 6 3D 1 4S 2 4P 3; c) 1s 2 2S 2 2P 6 3S 2 3P 6 3D 1 4S 2; d) 1S 2 2S 2 2P 6 3S 2 3P 6 3D 3 4S 2. |
|
Determine the element whose atom contains on the 4P-pylon 4 of the electron. What period and group is it? a) arsenic, IV period, VA group; b) Tellur, V Period, VIA Group; c) selenium, iv period, VIA group; d) Tungsten, VI period, VIB Group. |
Calcium and scandium atoms differ in each other: a) the number of energy levels; b) radius; c) the number of valence electrons; d) the formula of higher oxide. |
|
For sulfur and chromium atoms in the same way: a) the number of valence electrons; b) the number of energy levels; c) the highest valence; d) the formula of higher oxide. |
|
Nitrogen and phosphorus atoms have: a) the same number of electronic layers; b) the same number of protons in the kernel; c) the same number of valence electrons; d) identical radii. |
|
The formula of higher oxide of the element III period, in the atom of which is basically the state contains three unpaired electrons: a) e 2 o 3; b) EO 2; c) e 2 o 5; d) e 2 o 7. |
|
The formula of higher oxide element EO 3. Indicate the formula of its hydrogen compound: a) EN 2; b) en; c) EN 3; d) EN 4. |
|
The nature of oxides from the main to acid varies in the ranks: a) Na 2 O, MgO, SiO 2; b) Cl 2 O, SO 2, P 2 O 5, NO 2; c) beo, mgo, b 2 o 3, Al 2 O 3 ,; d) CO 2, B 2 O 3, Al 2 O 3, Li 2 O; e) SAO, Fe 2 O 3, Al 2 O 3, SO 2. |
|
Choose rows in which formulas are arranged in ascending order of acid properties of compounds: a) N 2 O 5, P 2 O 5, AS 2 O 5; c) H 2 SEO 3, H 2 SO 3, H 2 SO 4; b) hf, hbr, hi; d) Al 2 O 3, P 2 O 5, Cl 2 O 7. |
|
Specify a series in which hydroxides are arranged in order of increasing their main properties: a) lioh, koh, naoh; c) Lioh, Ca (OH) 2, Al (OH) 3; b) lioh, naoh, mg (oh) 2; d) Lioh, Naoh, Koh. |
Tasks
The phosphorus sample contains two nuclide: phosphorus-31 and phosphorus-33. The molar fraction of phosphorus-33 is 10%. Calculate the relative atomic mass of phosphorus in this sample.
Natural copper consists of Cu 63 and Cu 65 nuclides. The ratio of the number of Cu 63 atoms to the number of Cu 65 atoms in the mixture is 2.45: 1.05. Calculate the relative atomic mass of copper.
The average relative atomic mass of natural chlorine is 35.45. Calculate the molar fractions of its two isotopes, if it is known that their mass numbers are equal to 35 and 37.
The oxygen sample contains two nuclide: 16 o and 18 o, the masses of which are correspondingly 4.0 g and 9.0 g. Determine the relative atomic mass of oxygen in this sample.
The chemical element consists of two nuclides. The core of the first nuclide contains 10 protons and 10 neutrons. In the kernel of the second nuclide of neutrons by 2 more. On 9 atoms of lighter nuclide accounts for one atom of a heavier nuclide. Calculate the average atomic weight of the element.
What relative atomic mass would have oxygen if in the natural mixture for each 4 oxygen atom-16 accounted for 3 oxygen-17 atoms and 1 oxygen atom-18?
Answers:1. 31,2. 2. 63,6. 3. 35 CL: 77.5% and 37 CL: 22.5%. 4. 17,3. 5. 20,2. 6. 16,6.
Chemical communications
The main volume of educational material:
Nature and types of chemical bonds. The main parameters of the chemical bond: energy, length.
Covalent connection. Exchanged and donor-acceptor mechanisms for the formation of covalent communication. The direction and saturation of the covalent bond. Polarity and polarizability of a covalent bond. Valuation and degree of oxidation. Valental capabilities and valence conditions of atoms of elements of A-groups. Single and multiple links. Atomic crystal lattices. The concept of hybridization of atomic orbitals. The main types of hybridization. Corners of ties. The spatial structure of molecules. Empirical, molecular and structural (graphic) formula of molecules.
Ion communication. Ionic crystal lattices. Chemical formulas of substances with molecular, atomic and ionic structure.
Metal communication. Crystal metals lattices.
Intermolecular interaction. Molecular crystal lattice. Energy of intermolecular interaction and aggregate state of substances.
Hydrogen bond.The value of hydrogen bonds in natural objects.
As a result of the study, students should know:
what is a chemical relationship;
the main types of chemical bonds;
covalent Communication Education Mechanisms (exchange and donor-acceptor);
the main characteristics of the covalent bond (saturation, direction, polarity, multiplicity, s- and p-communications);
the main properties of ionic, metal and hydrogen bonds;
the main types of crystal lattices;
how the energy supply is changed and the nature of the molecules in the transition from one aggregate state to another;
what are distinguished by substances having a crystalline structure from substances having an amorphous structure.
As a result of studying the topic, students should acquire skills:
definitions of the type of chemical bond between atoms in various compounds;
comparison of the strength of chemical bonds by their energy;
determining the degrees of oxidation by the formulas of various substances;
establishment of the geometric shape of some molecules based on the theory of hybridization of atomic orbitals;
forecasting and comparing properties of substances depending on the nature of the relationship and the type of crystal lattice.
Having finished learning the topic, students should have a presentation:
- on the spatial structure of molecules (direction of covalent bonds, valence angle);
- On the theory of hybridization of atomic orbitals (SP 3 -, SP 2 -, SP-hybridization)
Having studied the topic, students should remember:
elements with a constant degree of oxidation;
compounds of hydrogen and oxygen in which these elements are not characteristic of them the degree of oxidation;
the magnitude of the angle between connections in the water molecule.
Section 1. Nature and types of chemical
Formulas of substances are given: Na 2 O, SO 3, KCl, PCl 3, HCl, H 2, Cl 2, NaCl, CO 2, (NH 4) 2 SO 4, H 2 O 2, CO, H 2 S, NH 4 CL, SO 2, HI, RB 2 SO 4, SR (OH) 2, H 2 SEO 4, HE, SCCL 3, N 2, ALBR 3, HBr, H 2 SE, H 2 O, OF 2, CH 4, NH 3, Ki, Cabr 2, Bao, NO, FCL, SIC. Select connections:
molecular and non-elastic structure;
only with covalent polar bonds;
only with covalent non-polar bonds;
only with ionic connections;
combining in the structure of ionic and covalent bonds;
combining covalent polar and covalent non-polar connections;
capable of forming hydrogen bonds;
having in structure of communication formed by donor-acceptor mechanism;
How does the polarity of connections in the ranks?
a) H 2 O; H 2 s; H 2 SE; H 2 TE b) pH 3; H 2 s; HCl.
In what state is the main or excited - there are atoms of isolated elements in the following connections:
B.Cl 3; P.Cl 3; SIO 2; BE.F 2; H 2. S.; C.H 4; H. Cl.O 4?
What a pair of these elements in chemical interaction has a maximum tendency to form ion communication:
Ca, C, K, O, I, CL, F?
In which of the chemicals proposed below, the bond rupture will be more likely to proceed with the formation of ions, and in which to form free radicals: NaCl, CS 2, CH 4, K 2 O, H 2 SO 4, KOH, CL 2?
Dana halogen breeds: HF, HCl, HBr, Hi. Choose a halogen garden:
aqueous solution of which is the strongest acid (the weakest acid);
with the most polar bond (the least polar bond);
with the highest length of communication (with the lowest communication length);
with the greatest boiling point (with the smallest boiling point).
When forming one chemical bond, fluoro fluoro is released 2.64 '
10 -19 J Energy. Calculate what chemical number of fluorine molecules should form in order to distinguish 1.00 kJ of energy.
Test 6.
When the molecule is formed from two isolated atoms, the energy in the system: a) increases; b) decreases; c) does not change; d) possible both a decrease and an increase in energy. |
|
Indicate, in what pair of substances, the general electronic pairs are shifted towards the oxygen atom: a) of 2 and co; b) Cl 2 O and NO; c) H 2 O and N 2 O 3; d) H 2 O 2 and O 2 F 2. |
|
Specify connections with a covalent non-polar connection: a) O 2; b) N 2; c) Cl 2; d) PCL 5. |
|
Specify connections with a covalent polar bond: a) H 2 O; b) br 2; c) CL 2 O; d) SO 2. |
|
Choose a couple of molecules, all links in which are covalent: a) NaCl, HCl; b) CO 2, Na 2 O; c) CH 3 Cl, CH 3 Na; d) SO 2, NO 2. |
|
Connections with covalent polar and covalent non-polar communication are respectively: a) water and hydrogen sulfide; b) bromide potassium and nitrogen; c) ammonia and hydrogen; d) oxygen and methane. |
|
None of the covalent bonds is formed by a donor-acceptor mechanism in a particle: a) CO 2; b) co; c) bf 4 -; d) NH 4 +. |
|
As the difference in the electrical negativeness of the bound atoms occurs: a) reduction of the polarity of communication; b) strengthening the polarity of communication; c) an increase in the degree of ionic communication; d) Reducing the degree of ionic communication. |
|
In which row of molecules are located in the order of increasing the polarity of communication? a) HF, HCl, HBr; b) NH 3, pH 3, ASH 3; c) H 2 SE, H 2 S, H 2 O; d) CO 2, CS 2, CSE 2. |
|
The greatest binding energy in the molecule: a) H 2 TE; b) H 2 SE; c) h 2 s; d) H 2 O. |
|
Chemical bond is the least durable in the molecule: a) bromomodorod; b) chloride; c) iododorodor; d) fluoroodorod. |
|
The length of the communication increases in a row of substances having formulas: a) CCL 4, CBR 4, CF 4; b) SO 2, SEO 2, TEO 2; c) H 2 S, H 2 O, H 2 SE; d) HBr, HCl, HF. |
|
Maximum numbers.- Communications that can exist between two atoms in the molecule: a) 1; b) 2; in 3; d) 4. |
|
The triple connection between the two atoms includes: a) 2 s-bonds and 1 π-bond; b) 3 s communication; c) 3 π-bonds; d) 1S communication and 2π-bonds. |
|
Molecule S. 2 Contains chemical links: a) 1s and 1π; b) 2s and 2π; c) 3s and 1π; d) 4s. |
|
Sums.- I.π- connections (s. + π) in moleculeSO. 2 Cl. 2 equal to: a) 3 + 3; b) 3 + 2; c) 4 + 2; d) 4 + 3. |
|
Specify connections with ion bond: a) sodium chloride; b) carbon oxide (II); c) iodine; d) Nitrate potassium. |
|
Only ionic communications support the structure of the substance: a) sodium peroxide; b) harated lime; c) copper sipop; d) Sylvinit. |
|
Indicate the atom of which element can participate in the formation of metal and ion communication: a) as; b) br; c) k; d) SE. |
|
The most pronounced character of ionic communication in the compound: a) calcium chloride; b) potassium fluoride; c) aluminum fluoride; d) sodium chloride. |
|
Specify the substances that the aggregate state of which under normal conditions is determined by hydrogen bonds between molecules: a) hydrogen; b) chloride; c) liquid fluorine garden; d) water. |
|
Specify the most durable hydrogen bond: a) -n .... H-; b) -o .... H-; c) -cl .... H-; d) -s .... H-. |
|
What chemical relationship is the least strong? a) metal; b) ionic; c) hydrogen; d) covalent. |
|
Specify the type of communication in the NF molecule 3 : a) ionic; b) non-polar covalent; c) polar covalent d) hydrogen. |
|
Chemical bond between the atoms of elements with sequence numbers 8 and 16: a) ionic; b) covalent polar; c) covalent non-polar; d) hydrogen. |
|
Periodic law.
Building atom
The article presents test tasks on the topic of test tasks drawn up by the authors for thematic control in the 8th grade. (Bank capacity - 80 tasks for each of the six topics studied in the 8th grade, and 120 tasks on the topic "Basic class of inorganic compounds".) Currently, chemistry training in the 8th grade is carried out on nine textbooks. Therefore, at the end of the article, a list of controlled elements of knowledge is given indicating task numbers. This will allow teachers working in different programs to choose both the appropriate sequence of tasks from one topic and a set of combinations of test tasks from different topics, including for final control.
The proposed 80 test tasks are grouped by 20 questions in four options, in which similar tasks are repeated. To compile more options from the list of knowledge elements, select (randomly) task numbers for each studied item in accordance with your thematic planning. Such a presentation of tasks for each topic allows you to spend a quick element analysis of errors and their timely correction. The use of similar tasks In one embodiment, the alternation of one or two correct answers reduces the likelihood of guessing the response. The complexity of the issues, as a rule, increases from the 1st and 2nd options to the 3rd and 4th options.
There is an opinion that the tests are "Guessing". We suggest you check if it is. After testing, compare the results with the marks. If the test results are below, it can be explained by the following reasons.
First, such (test) is unusual for students. Secondly, the teacher puts the emphasis in the study of the topic (determining the main in the content of education and teaching methodology).
Option 1
Tasks.
1. In the 4th period, VIA group is an element with a sequence number:
1) 25; 2) 22; 3) 24; 4) 34.
2. The element with the charge of the nucleus of the atom +12 has a sequence number:
1) 3; 2) 12; 3) 2; 4) 24.
3. The sequence number of the element corresponds to this characteristics:
1) the charge of the atom nuclei;
2) the number of protons;
3) the number of neutrons;
4. Six electrons at the external energy level at the atoms of elements with the group number:
1) ii; 2) iii; 3) VI; 4) IV.
5. The formula of higher oxide chlorine:
1) SL 2 O; 2) CL 2 O 3;
3) CL 2 O 5; 4) CL 2 O 7.
6. The valence of the aluminum atom is:
1) 1; 2) 2; 3) 3; 4) 4.
7. The total formula of volatile hydrogen compounds of the elements of the group VI:
1) EN 4; 2) EN 3;
3) NE; 4) H 2 E.
8. The number of the outer electronic layer in the calcium atom:
1) 1; 2) 2; 3) 3; 4) 4.
9.
1) Li; 2) Na; 3) k; 4) CS.
10. Specify elements-metals:
1) k; 2) Cu; 3) O; 4) N.
11. Where in Table D.I. Imeleeva, elements are located, whose atoms in chemical reactions only give electrons?
1) in group II;
2) at the beginning of the 2nd period;
3) in the middle of the 2nd period;
4) in the VIA group.
12.
2) ve, mg; AL;
3) Mg, Ca, SR;
13. Specify non-metallyle elements:
1) CL; 2) s; 3) Mn; 4) MG.
14. Non-metallic properties increase in a row:
15. What is the characteristics of the atom change periodically?
1) charge nucleus atom;
2) the number of energy levels in the atom;
3) the number of electrons in the external energy level;
4) Number of neutrons.
16.
1 TO; 2) AL; 3) p; 4) CL.
17. In a period with an increase in the charge of the kernel, the radii of atoms of elements:
1) decrease;
2) do not change;
3) increase;
4) change periodically.
18. The isotopes of atoms of one element differ by:
1) the number of neutrons;
2) the number of protons;
3) the number of valence electrons;
4) the provision in the table D.I. Imendeeva.
19. Number of neutrons in the kernel of atom 12 s:
1) 12; 2) 4; 3) 6; 4) 2.
20. The distribution of electrons by energy levels in the fluorine atom:
1) 2, 8, 4; 2) 2,6;
3) 2, 7; 4) 2, 8, 5.
Option 2.
Tasks. Select one or two correct answers.
21. The element with the sequence number 35 is in:
1) 7th period, IVA group;
2) 4th period, VIIA group;
3) 4th period, VIIB Group;
4) 7th period, IVB Group.
22. The element with the charge of the nucleus of the atom +9 has a sequence number:
1) 19; 2) 10; 3) 4; 4) 9.
23. The number of protons in the neutral atom coincides with:
1) the number of neutrons;
2) atomic mass;
3) the sequence number;
4) the number of electrons.
24. Five electrons at the external energy level at the atoms of elements with a group number:
1) i; 2) iii; 3) v; 4) VII.
25. The formula of higher nitrogen oxide:
1) N 2 O; 2) N 2 O 3;
3) N 2 O 5; 4) NO;
26. The valence of calcium atom in its highest hydroxide is equal to:
1) 1; 2) 2; 3) 3; 4) 4.
27. The valence of arsenic atom in its hydrogen compound is:
1) 1; 2) 2; 3) 3; 4) 4.
28. The number of the outer electronic layer in the potassium atom:
1) 1; 2) 2; 3) 3; 4) 4.
29. The greatest radius of the atom from the element:
1) b; 2) o; 3) C; 4) N.
30. Specify elements-metals:
1 TO; 2) H; 3) f; 4) Cu.
31. Atoms of elements capable of both taking and give electrons, are located:
1) in the IA group;
2) in the VIA group;
3) at the beginning of the 2nd period;
4) at the end of the 3rd period.
32.
1) NA, K, LI; 2) AL, MG, NA;
3) p, s, Сl; 4) Na, Mg, Al.
33. Specify non-metallyle elements:
1) Na; 2) MG; 3) Si; 4) P.
34.
35. The main characteristic of the chemical element:
1) atomic mass;
2) the charge of the core;
3) the number of energy levels;
4) Number of neutrons.
36. The symbol of the element whose atoms form amphoteric oxide:
1) n; 2) k; 3) s; 4) Zn.
37. In the main subgroups (a) of the periodic system of chemical elements with an increase in the charge of the nucleus, the atom radius:
1) increases;
2) decreases;
3) does not change;
4) varies periodically.
38. The number of neutrons in the nucleus of the atom is:
1) the number of electrons;
2) the number of protons;
3) the difference between the relative atomic mass and the number of protons;
4) atomic mass.
39. Hydrogen isotopes differ in the number:
1) electrons;
2) neutrons;
3) protons;
4) position in the table.
40. The distribution of electrons by energy levels in the sodium atom:
1) 2, 1; 2) 2, 8, 1;
3) 2, 4; 4) 2, 5.
Option 3.
Tasks. Select one or two correct answers.
41. Specify the sequence number of the element, which is located in the IVA group, the 4th period of the table D.I. Iveleeva:
1) 24; 2) 34; 3) 32; 4) 82.
42. The charge of the nucleus of an atom of element number 13 is:
1) +27; 2) +14; 3) +13; 4) +3.
43. The number of electrons in the atom is:
1) the number of neutrons;
2) the number of protons;
3) atomic mass;
4) the sequence number.
44. Atoms of the IVA elements of the group, the number of valence electrons is:
1) 5; 2) 6; 3) 3; 4) 4.
45. Oxides with the general formula R 2 o 3 form elements of the series:
1) Na, K, Li; 2) MG, CA, BE;
3) B, Al, GA; 4) C, SI, GE.
46. The valence of the phosphorus atom in its highest oxide is:
1) 1; 2) 3; 3) 5; 4) 4.
47. Hydrogen compounds of elements of the group VIIA:
1) HCLO 4; 2) HCl;
3) HBro; 4) HBr.
48. The number of electronic layers in the Selena atom is:
1) 1; 2) 2; 3) 3; 4) 4.
49. The greatest radius of the atom from the element:
1) Li; 2) Na; 3) Mg;
50. Specify elements-metals:
1) Na; 2) MG; 3) Si; 4) P.
51. Atoms of which elements easily give electrons?
1) k; 2) Cl; 3) Na; 4) S.
52. A number of elements in which metal properties increase:
1) C, N, B, F;
2) al, si, p, mg;
53. Specify non-metallyle elements:
1) Na; 2) MG; 3) n; 4) S.
54. A number of elements in which non-metallic properties increase:
1) li, na, k, n;
2) AL, SI, P, MG;
3) C, N, O, F;
4) Na, Mg, Al, K.
55. With an increase in the charge of the nucleus of the atom, the non-metallic properties of the elements:
1) change periodically;
2) amplified;
3) do not change;
4) weaken.
56. The symbol of the element whose atoms form amphoteric hydroxide:
1) Na; 2) Al; 3) n; 4) S.
57. The frequency of changing the properties of elements and their compounds is explained:
1) the repetition of the structure of the outer electronic layer;
2) an increase in the number of electronic layers;
3) an increase in the number of neutrons;
4) an increase in atomic mass.
58. The number of protons in the nucleus of the sodium atom is:
1) 23; 2) 12; 3) 1; 4) 11.
59. What is the difference between the atoms of the isotopes of one element?
1) the number of protons;
2) the number of neutrons;
3) the number of electrons;
4) the charge of the kernel.
60. The distribution of electrons by energy levels in the lithium atom:
1) 2, 1; 2) 2, 8, 1;
3) 2, 4; 4) 2, 5;
Option 4.
Tasks. Select one or two correct answers.
61. The element with the sequence number 29 is in:
1) 4th period, IA group;
2) 4th period, the IB group;
3) 1st period, IA group;
4) 5th period, IA group.
62. The charge of the nucleus of an atom of element No. 15 is:
1) +31; 2) 5; 3) +3; 4) +15.
63. The charge of the atom nucleus is determined by:
1) the sequence number of the element;
2) group number;
3) the period number;
4) atomic mass.
64. At the atoms of the III elements of the group, the number of valence electrons is:
1) 1; 2) 2; 3) 3; 4) 5.
65. The highest sulfur oxide has a formula:
1) H 2 SO 3; 2) H 2 SO 4;
3) SO 3; 4) SO 2.
66. Formula of higher phosphorus oxide:
1) p 2 o 3; 2) H 3 PO 4;
3) NRU 3; 4) P 2 O 5.
67. Valuation of the nitrogen atom in its hydrogen compound:
1) 1; 2) 2; 3) 3; 4) 4.
68. The period number in Table D.I. REMEELEEVA corresponds to the following characteristic of the atom:
1) the number of valence electrons;
2) higher valence in compound with oxygen;
3) the total number of electrons;
4) the number of energy levels.
69. The greatest radius of the atom from the element:
1) CL; 2) br; 3) I; 4) F.
70. Specify elements-metals:
1) MG; 2) Li; 3) h; 4) S.
71. At the atom of which element is easier to give an electron?
1) sodium; 2) cesium;
3) potassium; 4) lithium.
72. Metal properties increase in a row:
1) Na, Mg, Al; 2) NA, K, RB;
3) RB, K, NA; 4) p, s, cl.
73. Specify non-metallyle elements:
1) Cu; 2) in; 3) N; 4) CR.
74. N-P-AS-SB non-metallic properties:
1) decrease;
2) do not change;
3) increase;
4) decrease, and then increase.
75. What characteristics of the atom change periodically?
1) relative atomic mass;
2) the charge of the core;
3) the number of energy levels in the atom;
4) the number of electrons at the external level.
76. Atoms of which element form amphoteric oxide?
1 TO; 2) ve; 3) C; 4) Sa.
77. In the period with an increase in the charge of the atom nucleus increases the attraction of electrons to the kernel and metal properties:
1) amplified;
2) change periodically;
3) weaken;
4) do not change.
78. The relative atomic mass of the element is numerically equal to:
1) the number of protons in the core;
2) the number of neutrons in the kernel;
3) the total number of neutrons and protons;
4) the number of electrons in the atom.
79. The number of neutrons in the nucleus of an atom 16 o is:
1) 1; 2) 0; 3) 8; 4) 32.
80. The distribution of electrons by energy levels in the silicon atom:
1) 2, 8, 4; 2) 2, 6;
3) 2, 7; 4) 2, 8, 5.
List of controlled elements of knowledge on the topic
"Periodic law. The structure of the atom
(In brackets are given through job numbers)
The sequence number of the element (1, 3, 21, 41, 61), the charge of the nucleus of the atom (2, 22, 42, 62, 63), the number of protons (23) and the number of electrons (43) in the atom.
Group number, electrons in the external energy level (4, 24, 44, 64), higher oxide formulas (5, 25, 45, 65), the highest valence of the element (6, 26, 46, 66), the formulas of hydrogen compounds (7 , 27, 47, 67).
Period number, number of electronic levels (8, 28, 48, 68).
Change the radius of an atom (9, 17, 29, 37, 49, 67, 69).
Position in Table D.I. REMEFELEEVA elements-metals (10, 30, 50, 70) and non-metal elements (13, 33, 53, 73).
The ability of atoms to give and accept electrons (11, 31, 51, 71).
Changing the properties of simple substances: by groups (12, 14, 34, 52, 54, 74) and periods (32, 72, 77).
Periodic change in the electronic structure of atoms and properties of simple substances and their compounds (15, 35, 55, 57, 75, 77).
Amphoteri oxides and hydroxides (16, 36, 56, 76).
Mass number, the number of protons and neutrons in the atom, isotopes (18, 19, 38, 39, 58, 59, 78, 79).
The distribution of electrons by energy levels in the atom (20, 40, 60, 80).
Answers to test tasks on the topic
"Periodic law. The structure of the atom
| Option 1 | Option 2. | Option 3. | Option 4. | ||||
|---|---|---|---|---|---|---|---|
| Quest Number | Response number | Quest Number | Response number | Quest Number | Response number | Quest Number | Response number |
| 1 | 4 | 21 | 2 | 41 | 3 | 61 | 2 |
| 2 | 2 | 22 | 4 | 42 | 3 | 62 | 4 |
| 3 | 1, 2 | 23 | 3, 4 | 43 | 2, 4 | 63 | 1 |
| 4 | 3 | 24 | 3 | 44 | 4 | 64 | 3 |
| 5 | 4 | 25 | 3 | 45 | 3 | 65 | 3 |
| 6 | 3 | 26 | 2 | 46 | 3 | 66 | 4 |
| 7 | 4 | 27 | 3 | 47 | 2, 4 | 67 | 3 |
| 8 | 4 | 28 | 4 | 48 | 4 | 68 | 4 |
| 9 | 4 | 29 | 1 | 49 | 5 | 69 | 3 |
| 10 | 1, 2 | 30 | 1, 4 | 50 | 1, 2 | 70 | 1, 2 |
| 11 | 1, 2 | 31 | 2, 4 | 51 | 1, 3 | 71 | 2 |
| 12 | 3 | 32 | 2 | 52 | 3 | 72 | 2 |
| 13 | 1, 2 | 33 | 3, 4 | 53 | 3, 4 | 73 | 2, 3 |
| 14 | 1 | 34 | 4 | 54 | 3 | 74 | 1 |
| 15 | 3 | 35 | 2 | 55 | 1 | 75 | 4 |
| 16 | 2 | 36 | 4 | 56 | 2 | 76 | 2 |
| 17 | 1 | 37 | 1 | 57 | 1 | 77 | 3 |
| 18 | 1 | 38 | 3 | 58 | 4 | 78 | 3 |
| 19 | 3 | 39 | 2 | 59 | 2 | 79 | 3 |
| 20 | 3 | 40 | 2 | 60 | 1 | 80 | 1 |
Literature
Gorodnichev I.N.. Control and verification of chemistry. M.: Aquarium, 1997; Sorokin V.V., Zlotnikov E.G. Chemistry tests. M.: Education, 1991.
It was mentioned above (p. 172) on the periodicity of changes in the most important properties of atoms - valence. There are other important properties whose change is characterized by frequency. Such properties include the size (radius) of the atom. Atom has no surfacesand the border is vague, as the density of external electron clouds as it removes it smoothly decreases. Data on atom radii is obtained from the determination of distances between their centers in molecules and crystalline structures. Calculations were also carried out on the basis of the equations of quantum mechanics. In fig. 5.10
Fig. 5.10. The frequency of changes in atomic radii
the curve of changes in atomic radii, depending on the charge of the kernel.
From hydrogen to helium, the radius decreases, and then sharply increases in lithium. This is due to the advent of an electron at the second energy level. In the second period from lithium to neo, as the charge of the kernel increases, radii decreases.
At the same time, an increase in the number of electrons in this energy level leads to an increase in their mutual repulsion. Therefore, by the end of the period, the decrease in the radius slows down.
When moving from neon to sodium - the first element of the third period - the radius again increases sharply, and then gradually decreases to argon. After that, there is a sharp increase in the radius in potassium. It turns out a characteristic periodic sawy curve. Each section of the alkali metal curve to a noble gas characterizes the change in the radius in the period: there is a decrease in the radius during the transition from left to right. It is also interesting to find out the nature of the change in radii in groups of elements. To do this, you need to hold a line through the elements of the same group. According to the position of the maxima in alkali metal, it is directly seen that the radii of atoms during the transition in the group from top to bottom increases. This is due to the increase in the number of electronic shells.
task 5.17. How do the radii of atoms from f to ur change? Determine this in fig. 5.10.
Many other properties of atoms are dependent on radii, both physical and chemical. For example, an increase in the radii of atoms can explain the decrease in alkali metal melting temperatures from lithium to cesium:
The sizes of atoms are associated with their energy properties. The greater the radius of external electron clouds, the easier it is an electron loses an electron. At the same time, it turns into a positively charged and he.
Ion is one of the possible states of the atom in which it has an electrical charge due to the loss or addition of electrons.
The ability of an atom to move into a positively charged ion is characterized energy of ionization E I.This is the minimum energy required for the separation of an external electron from an atom in the gas state:
![]()
The resulting positive ion can also lose electrons, becoming two-chain, three-chart, etc. The magnitude of the ionization energy is greatly increasing.
The ionization energy of atoms increases in the period when moving from left to right and decreases in groups when transition from top to bottom.
Many, but not all atoms are able to attach an additional electron, turning into a negatively charged ion A ~. This property is characterized electron Energy E Energy E cf. This is the energy distinguished when the electron is connected to the atom located in the gas state:
![]()
Both the energy of ionization and the energy of the germination of the electron is customary to 1 mole atoms and express in KJ / mol. Consider the ionization of the sodium atom as a result of attachment and loss of an electron (Fig. 5.11) . From the figure it is clear that for the exclusion of an electron from the sodium atom is required in 10 once more energy than it is allocated when an electron is connected. Negative sodium ion unstable and in complex substances is almost never found.

Fig. 5.11. Ionization of sodium atom
The ionization energy of atoms changes in periods and groups in the direction opposite to the change in the radius of atoms. The change in the energy of affinity to the electron in the period is more difficult, since the elements of the IIA- and VIIIA-RPYNN affinity is absent. We can approximately assume that the energy of an electron affinity is like E toincreases in periods (to group VII inclusive) and decreases in groups from top to bottom (Fig. 5.12).
the task 5 .eighteen. Can Magnesium and Argon atoms in a gaseous state of forming negatively charged ions?
Ions with positive and negative charges are attracted among themselves, which leads to a variety of transformations. The simplest case is the formation of ionic connections, i.e., unification of ions in a substance under the action of electrostatic attraction. Then the ionic crystal structure occurs, characteristic of the NaCl food salt and many other salts. But maybe

Fig. 5.12. The nature of changes in the energy of ionization and energy of an electron affinity in groups and periods
so, the negative ion is not very firmly holding its excess electron, and a positive ion, on the contrary, seeks to restore its electronterability. Then the interaction between ions can lead to the formation of molecules. Obviously, the ions of the different sign of the charge C1 + and C1 ~ are attracted among themselves. But due to the fact that these are ions of identical atoms, they form a C1 2 molecule with zero charges on atoms.
Questions and exercises
1. From how many protons, neutrons and electrons consist of bromine atoms?
2.
Calculate the mass fractions of isotoping nature. ![]()
3. How much energy is released during formation 16 g.oxygen by reaction ![]() flowing in depths of stars?
flowing in depths of stars?
4. Calculate the electron energy in an excited hydrogen atom when n \u003d3.
5. Write the complete and abbreviated electronic formulas of the iodine atom.
6. Write a reduced electronic formula of ion.
7. Write complete and abbreviated electronic formulas atom and iona 2.
8. Build energy diagrams of phosphorus and arsenic atoms.
9. Build full energy diagrams of zinc and gallium atoms.
10. Place the following atoms in the order of increasing the radius: aluminum, boron, nitrogen.
11. Which of the following ions are forming ionic crystal structures among themselves: in R + R -, K +, K -, I +, I -, LI +, LI -? What can be expected when the interaction of ions in other combinations?
12. Suppose the possible nature of the radius of atoms during the transition in the periodic system in the diagonal direction, for example Li - Mg - SC.