Ion exchange. Ionites and their classification
Exchange Capacity
For the quantitative characteristics of the ion exchange and sorption properties of ionovis, the following values \u200b\u200bare used: full, dynamic and working exchange tank.
Full exchange tank(PO) Determined by the number of functional groups capable of ion exchange, in a unit of mass of air-dry or swollen ionic and is expressed in MM-eq / g or ME-EQ / L. It is a constant value indicated in the ionate passport, and does not depend on the concentration or nature of the exchanging ion. This may vary (decrease) due to thermal, chemical or radiation impact. In real conditions, it decreases with time due to the aging of the ionate matrix, the irreversible absorption of poisone ions (organic, iron, etc.), which block functional groups.
Equilibrium (static) exchange capacity depends on the concentration of ions in water, pH and the ratio of ionate volumes and the solution at measurements. Need to carry out the calculations of technological processes.
Dynamic exchange tank(Daily) - the most important indicator in water treatment processes. In real conditions, the multiple use of ionet in the cycle of sorption-regeneration, the exchange capacity is not fully used, but only partially.
The degree of use is determined by the regeneration and flow rate of the regenerating agent, the time of contact of ionate with water and with a regenerating agent, the concentration of salts, pH, design and hydrodynamics of the device used. The figure shows that the process of water purification is stopped at a certain concentration of the limiting ion, as a rule, long before the full saturation of ionet. The number of ions absorbed in this case, corresponding to the area of \u200b\u200bthe rectangle A, assigned to the volume of ionet, and will be daily.
The number of absorbed ions corresponding to the complete saturation, when the spock is 1, corresponding to the amount of the amount and the area of \u200b\u200bthe shaded figure above the S-like curve, is called the total dynamic exchange capacity (PD). In type-type water treatment processes, it usually does not exceed 0.4-0.7 in.
Fig. one
experimental part
Reagents and solutions:mgCl2 * 6H2O salts in distilled water in a measuring flask with a capacity of 250 cm
The solution of calcium nitrate (0.02 m) was prepared by dissolving the sample (1.18 g.) Sali Ca (NO3) 2 · 4N20. After dissolving the sample, the solution was diluted in distilled water in a measuring flask with a capacity of 250 cm.
Solution 2 calcium nitrate (O.1m) was prepared by dissolving the sample (5.09 g.) Salts of Ca (NO3) 2 · 4N20. After dissolving the sample, the solution was diluted in distilled water in a measuring flask with a capacity of 250 cm.
Original solution of complexone IIIprepared from fixanal. Standardization was performed according to magnesium sulfate.
Buffer solutions prepared from NH4Cl "Ch.D.A." and NH4OH.
The residual concentration of Mg 2+ ions was determined by complexenometrically with the Eryoichroma Indicator T.
The residual concentration of Ca 2+ ions was determined by complexenometrically with the Murcexide indicator.
The sorbed concentration was found in terms of the initial and residual.
As a sorbent was used by the Zeolites-containing breed of the Artyhevsky manifestation.
Cooking the sorbent.
CSP ATHESHEVSKY manifestation of crushing, sieved, collected fractions of granules with a size of 1 - 2 - 3 mm and dried in a drying cabinet.
Ion exchange container in static mode. By 20 cm with a solution containing Ca 2+ ions, in another case, Mg 2+, with a known concentration and
a certain pH value was added 5.0 g of the sorbent, shaken for a specified time and separated the solid phase by filtration. IN
The selectivity of chelametometric titration with regard to calcium can be improved by determining the determination in a strongly alcohol medium (the magnesium filtrate was determined by the residual concentration of Ca 2+ ions, in the other case, Mg 2+. Surface concentration was found in terms of the source and residual.
Metal chromic indicator - Muriesid.
EDTA, 0.05m solution; ammonium buffer mixture pH \u003d 9; NaOH, 2m solution; Indicators - Eryoichrom Black T and Muriesid - solid (mixture with NaCl in terms of 1: 100).
Methodology Definition
1. The sample of the analyzed solution was transferred to the titration flask, 10cm 3 ammonium buffer mixture (pH 9), 25 cm 3 of distilled water was added, on the tip of a spatula 30 - 40 mg ERIOHROM of the Black T and outweigh the system to completely dissolve the indicator. The solution acquired wine-red. The titration of EDTA solution was performed dropwise from a burette with continuous stirring to the color of the color in clearly blue.
2. The sample of the analyzed solution was transferred to the flask for titration, add 5 cm 3 2 m NaOH solution, 30 cm 3 of distilled water and on the tip of a spatula of 30 mGExide. The solution acquired a red color. Titration was performed by a solution of EDTA before the transition of the color in purple.
Calculation of statistical conditions in relation to calcium and magnesium ions.
Determination of Magnesium Exchange Capacity
By 20 cm 3 Magnesium chloride solution with a molar concentration of equivalent 0.02 mol / l added and 5.0 s sorbent pre-dried at 105 0 s for 1 hour and shake for a specified time (0.5 hours). In another case, 1 hour and so on. After time expired, the solution was filtered. It was taken to analyze 5 cm 3 of the filtrate and the residual concentration of Mg 2+ ions was determined by the complexionometric method.
2. By 20 cm3 of the calcium chloride solution with a molar concentration of equivalent 0, l mole / l added 5.0 g of the sorbent pre-dried at 1050C for 1 hour and shake for a specified time (0.5 hours). In another case, 1 hour and so on. After time expired, the solution was filtered. It was taken to analyze 5 cm3 of the filtrate and the residual concentration of Ca2 ions was determined by the complexionometric method.
The influence of the contact time of the CSP and the solution CaCl2 * 4N2O on the exchange tank of the CSP in static conditions.
(C (Ca2 +) Ex \u003d 0.1 mol / l; MCSP \u003d 5.0)
With an increase in the contact time of the phases, an increase in equilibrium concentration is observed. And after 3 hours, a dynamic rolling equilibrium is installed.
6. Restriction of the validity of the Discontinued Protocol No. 5-94 of the Interstate Council on Standardization, Metrology and Certification (IUS 11-12-94)
7. Edition (January 2002) with amendment (IUS 3-91)
This standard applies to ionites and establishes methods for determining dynamic exchange tank with full ionic regeneration and with a given regenerating agent consumption.
Methods consist in determining the number of ions are absorbed from the working solution by a unit of the volume of swollen ionite with a continuous flow of the solution through the ionite layer.
1. Sampling method
1. Sampling method
1.1. The sampling method indicates the regulatory and technical documentation for specific products.
1.2. For ionites, in which the mass fraction of moisture is less than 30%, the sample is taken (100 ± 10). For swelling, the sample is placed in a glass with a capacity of 600 cm and is poured with a saturated solution of sodium chloride, which should be coated with an excess to cover the ionite layer with its swelling. After 5 h ionis is washed with distilled water.
1.3. For ionites with a mass fraction of moisture, more than 30% is taken by a sample (150 ± 10) g into a glass of 600 cm with a capacity and 200 cm of distilled water is adhered.
2. Reagents, solutions, dishes, devices
Water distilled according to GOST 6709 or demineralized, meets the requirements of GOST 6709.
Barium chloride according to GOST 742, H.Ch., solution with a mass fraction of 10%.
Calcium 2-water chloride, H.Ch., concentration solutions (Sasl \u003d 0.01 mol / dm (0.01 n.) And (sasl) \u003d 0.0035 mol / dm (0.0035 n.).
Salonic acid according to GOST 3118, H.Ch., solutions with a mass fraction of 5% and concentrations (HCl) \u003d 0.5 mol / dm (0.5 n.), (HCl) \u003d 0.1 mol / dm (0, 1 n.) And (nsl) \u003d 0.0035 mol / dm (0.0035 n.).
Sulfuric acid according to GOST 4204, H.Ch., solutions with a mass fraction of 1%, concentration (HSO) \u003d 0.5 mol / dm (0.5 n.).
Sodium hydroxide according to GOST 4328, H.Ch., solutions with a mass fraction of 2, 4, 5%, concentrations (NaOH) \u003d 0.5 mol / dm (0.5 n.), (NaOH) \u003d 0.1 mol / dm (0.1 n.), (NaOH) \u003d 0.0035 mol / dm (0.0035 n.).
Sodium chloride according to GOST 4233, H.C., saturated solution and a solution of concentration (NACI) \u003d 0.01 mol / dm (0.01 n.).
The mixed indicator consisting of methyl red and methylene blue or methyl red and bromon-green green is prepared according to GOST 4919.1.
Methyl orange or methyl red indicator, a solution with a mass fraction of 0.1% is prepared according to GOST 4919.1.
Phenolphthalein indicator, alcohol solution with a mass fraction of 1%, prepare according to GOST 4919.1.
The absorber chemical lime CPI-1 according to GOST 6755 or the lime is natron.
Tube (Hlorkalcium) according to GOST 25336.
Menzur 1000 according to GOST 1770.
Cylinders according to GOST 1770 performances 1-4 with a capacity of 100 and 250 cm and performances 1, 2 with a capacity of 500 and 1000 cm.
Glasses in or n according to GOST 25336 in any performance with a capacity of 600 and 1000 cm.
Flasks KN-1-250 according to GOST 25336.
Pipettes 2-2-100, 2-2-25, 2-2-20 and 2-2-10 via NTD.
Burettes on NTD types 1, 2, performances 1-5, accuracy classes 1, 2, with a capacity of 25 or 50 cm, with a division price of not more than 0.1 cm and burets of types 1, 2, execution 6, accuracy classes 1, 2, With a capacity of 2 or 5 cm, with a division price of not more than 0.02 cm.
The measuring flasks 1, 2 according to GOST 1770, accuracy classes 1, 2, with a capacity of 10, 25 and 100 cm.
Sieve with a control grid 0315K according to GOST 6613 with a shell with a diameter of 200 mm.
The CCC-5000 cup according to GOST 25336 or from a polymerization material sufficient to place the sieve into it.
Installation laboratory (see the drawing) consists of a bottle 1 and a glass column with a 6 inner diameter (25.0 ± 1.0) mm and a height of at least 600 mm to determine the dynamic exchange capacity under conditions of full ionic regeneration and inner diameter (16.0 ± 0.5) mm and a height of at least 850 mm to determine under conditions of a specified flow of regenerating agent. In the lower part of the column of the WPIAN filter 7 of the POR 250 POR 250 xc according to GOST 25336 or the other filtering device, resistant to acids and alkalis, not transmitting ionis grains of more than 0.25 mm and having a small filtering resistance. The column is connected to a bottle with a glass tube 3 and a rubber hose 4 with a screw clip 5. To prevent carbon dioxide from air from air into a solution of sodium hydroxide into a bottle tube, a chloricalcium tube 2 is installed with the HPP-1 absorber.
Laboratory installation
It is allowed to use other measuring instruments with metrological characteristics not worse than those mentioned, as well as the quality reagents not lower than the specified.
3. Method for determining dynamic exchange capacity with full ionic regeneration
3.1. Preparation for the test
3.1.1. Preparation for testing is carried out according to GOST 10896 and after training ion is stored in a closed flask under the layer of distilled water.
Kaotionitis Ku-2-8 hp brand and the anion of the brand AB-17-8Cs to the test according to GOST 10896 are not prepared.
3.1.2. The ionic sample from the flask in the form of an aqueous suspension is transferred to a cylinder with a capacity of 100 cm and seal a layer of ionate by tapping about the solid surface of the bottom of the cylinder to the cessation of shrinkage. The volume of ion is adjusted to 100 cm and with the help of distilled water tolerate iones into the column, following the air bubbles between the ionics granules. Excess water from the column is drained, leaving 1-2 cm layer over the ionite level.
3.1.3. The iones in the column is washed with distilled water, passing it from top to bottom at a speed of 1.0 dm / h. At the same time, anionitis is washed from alkali (according to phenolphthalene), and the acid cation (according to methyl orange).
3.1.4. Highly binding anions in hydroxyl form are quickly loaded and washed with water that does not contain carbon dioxide.
3.2. Testing
3.2.1. The determination of the dynamic exchange capacity of iones consists of several cycles, each of which includes three consecutive operations - saturation, regeneration, washing, the conditions of which are shown in Table 1.
Table 1
Conditions for determining the dynamic exchange capacity with the full ionic regeneration
Indicator | Ionit class | Solution for saturation of ionites | Saturation control | Regeneration | |||
satisfying | laundry | regeneration |
|||||
Dynamic exchange capacity to slippath () | Strong- | Calcium chloride (CaCl) \u003d 0.01 mol / dm (0.01 n.) | Before the concentration of calcium ions in the filtrate (Ca) \u003d 0.05 mmol / dm (0.05 mg · eq / dm) are determined by GOST 4151 | Hydrochloric acid, solution with a mass fraction of 5% | |||
Strong- | Sodium chloride (NaCl) \u003d 0.01 mol / dm (0.01 n.) | Prior to a decrease in the concentration of alkali by 0.5 mmol / dm (0.5 mg · eq / dm) in comparison with the maximum stable value in the filtrate [Mixed indicator, title solution, concentration hydrochloric acid (HCl) \u003d 0.01 mol / dm (0.01 n.)] And before increasing the content of chlorine ions in comparison with its sustainable content in the filtrate (defined according to GOST 15615) | Sodium hydroxide, solution with a mass fraction of 5% | ||||
Weakly | Before the appearance in the filtrate of acids (by methyl orange) | ||||||
Complete dynamic exchange capacity () | Weakly | Salonic acid (HCl) \u003d 0.1 mol / dm (0.1 n.) | Prior to equalization of the filtrate concentration with the concentration of the working solution | Sodium hydroxide, solution with a mass fraction of 2% | |||
Notes:
1. When determining the concentration of Ca ions according to GOST 4151
2. The specific load is the volume of the solution passed through the ionet volume per 1 hour. For example, 5 dm / dm · h corresponds to the filtration rate at which it is 100 cm of solution (8.3 cm / min) after 100 cm of ionite .
3. The filtering rate is set to measure in a measuring cylinder of the filtrate volume obtained during a certain time interval.
Solutions and water are fed from top to bottom. When enjoying the anonita brands of the An-1 and the AN-2FN solutions are reduced from below.
3.2.2 Before conducting saturation, regeneration and washing operations, the column is filled with an appropriate solution. The solution layer over ion should be (15 ± 3) cm.
3.2.3. After saturation, regeneration and washing in the column over ion, leave a layer of liquid with a height of 1-2 cm.
3.2.4. The ionic column is filled with the working solution for a specific class of ionite (see Table 1) so that the layer of solution over ion is (15 ± 3) cm, and select the corresponding filtration rate.
When the concentration of 0.1 mol / dm is passed through a column with an ion (0.1 n.), The filtrate is collected in cylinders with a capacity of 250 cm, at a concentration of 0.01 mol / dm (0.01 n.) - in cylinders with a capacity 1000 cm. In the second and subsequent saturation cycles before the appearance of the working solution ions in the filtrate (determined after the first cycle), the filtrate is collected by portions of 100 and 250 cm according to the concentrations of the working solution.
3.2.5. From each portion of the filtrate, the sample is taken and controls the saturation in accordance with Table 1.
3.2.6. After appearing in the portion of the filtrate, the ions of the working solution calculate the total filtrate volume.
3.2.7. To determine the complete dynamic exchange capacity, the solution is continued to align the filtrate concentration with the concentration of the working solution. The saturation control in this case is carried out by the titration of the sample with a solution of an acid (sodium hydroxide) with a mixed indicator before changing the color.
3.2.8. Before carrying out the regeneration, ionites in the column explode the current of distilled water from the bottom up so that all the ionth grains are in motion. Explosion of the KU-1 brand cationia and the aniones of the brands of the An-1 and An-2FN are carried out before the saturation operation.
3.2.9. The ionic regeneration is carried out with a solution of acid (sodium hydroxide) at the rate specified in Table 1. The filtrate is continuously assembled by a cylinder with a cylinder with a volume of 250-1000 cm, adding 3-4 drops of the indicator. When an acid appears (sodium hydroxide), its concentration is determined in the filtrate in the subsequent portions. To control the filtrate, the sample is taken with a pipette or measurable flask and titrate with a solution of acid (sodium hydroxide) concentration (HSL, HSO) \u003d 0.5 mol / dm (0.5 n.), (NaOH) \u003d 0.5 mol / dm (0 , 5 n.) In the presence of the indicator
3.2.10. The acid solution (sodium hydroxide) is passed to equalizing the concentration of the filtrate with the concentration of the regenerating solution.
3.2.11. The ionis after regeneration is washed with distilled water to a neutral reaction according to methyl orange (phenolphthalene) at a speed specified in Table 1. Then the ion is kept in distilled water for 1 hour and the filtrate check again. If the filtrate does not have a neutral reaction, the ion is washed again.
3.2.12. The definition of a dynamic exchange capacity is complete if the last cycles obtained results, the discrepancy between which does not exceed 5% of the average result.
3.2.13. The dynamic exchanging capacity of the anion of AB-17-8CC is determined by two parallel samples on the first saturation cycle, before the appearance of the working solution ions in the filtrate. The filtrate is collected by portions of 250 cm. For the result, the average arithmetic results of two definitions, the allowable discrepancy between which does not exceed 5% of the average result.
(Amendment, IUS 3-91).
4. Method for determining dynamic exchange containers with a given regenerating consumption
4.1. Preparation for the test
4.1.1. The ionis, selected in accordance with claims 1.2 and 1.3, is separated from small fractions by the method of wet resusual according to GOST 10900, using a sieve with a grid N 0315K.
4.1.2. The separated anionite is placed in a glass, 500 cm of sodium hydroxide solution with a mass fraction of 4% are stirred and stirred. After 4 h, the hydroxide solution is drained, and the anion is washed with water to a slightly alkaline reaction according to phenolphthalene and transferred to the column, as indicated in clause 3.1.2.
4.1.3. The separated cation is washed from suspension and mutted with distilled water with decantation before the appearance of light wash water and transferred to the column in accordance with clause 3.1.2.
4.2. Testing
4.2.1. Determination of the dynamic exchange tank of ionites before the appearance of the working solution ions in the filtrate () consists of several cycles, each of which includes three consecutive operations - saturation, regeneration, washing, whose conditions are shown in Table 2. Solutions and water are fed from top to bottom. The height of the liquid layer above the ionet level is set as specified in PP.3.2.2 and 3.2.3.
table 2
Conditions for determining the dynamic exchange capacity of iones at a given regenerating consumption
Ionit class | Regeneration | Specific value rate regenerated | Wash control | Ionite saturation solution | Saturation control | Filtration speed |
||
nasa | fucking | registers |
||||||
Strong | To the residual concentration of acid in the filtrate no more | Calcium chloride (sasl \u003d 0.0035 mol / dm (0.0035 n.) | Before the concentration of calcium ions in the filtrate more (Ca) \u003d 0.05 mmol / dm | |||||
Weakly | Sulfuric acid, solution with a mass fraction of 1% | Until the absence in the sulfation filtrate (sample with bacll in the presence of HCl) | Sodium hydroxide (NaOH) \u003d 0.0035 mol / dm (0.0035 n.) | Up to concentration in sodium hydroxide filtrate (NaOH) \u003d 0.1 mmol / dm | ||||
Strong- | Sodium hydroxide with a mass fraction of 4% | To the residual concentration of sodium hydroxide in the filtrate no more (NaOH) \u003d 0.2 mmol / dm | Sodium chloride (NACI) \u003d 0.01 mol / dm (0.01 n.) | Prior to a decrease in alkali concentration on (NaOH) \u003d 0.7 mmol / dm | ||||
Weakly | Sodium hydroxide, solution with a mass fraction of 4% | To the residual concentration of sodium hydroxide in the filtrate not more (NaOH) \u003d 0.2 mmol / dm (0.2 mg · eq / dm) via phenolphthalene | Salt (sulfuric) acid (HSL, HSO) \u003d 0.0035 mol / dm (0.0035 n.) | Up to the residual concentration of acid in the filtrate is not more (H) \u003d 0.1 mmol / dm (0.1 mg · eq / dm), the indicator is mixed, the title solution - sodium hydroxide concentration (NaOH) \u003d 0.01 mol / dm (0 , 01 n.) | ||||
Notes:
1. When expressing the norm of specific flow rate of the regenerating agent () in grams on the mole under the word "mole" mean the molar mass of the ion equivalent (NA, K, CA, MG, CL, NO, NSO, HSO, CO, SO
Etc.).
2. The actual flow rate of the regenerating agent should not differ from the specified norm by more than 5%.
3. When determining the concentration of SA ions according to GOST 4151, use of 2-3 drops of the indicator of chrom-dark blue and titration of trilone-b concentration (NaHCon · 2HO) \u003d 0.01 mol / dm (0.01
4. The specific load is the volume of the solution passed through the volume of ionis per 1 hour. For example, 5 dm / dm · h corresponds to the filtration rate at which it is 100 cm of solution (8.3 cm / min) after 100 cm of ionite .
5. Filtration rate is set to measure in a measuring cylinder of the filtrate volume obtained during a certain time interval.
In order to avoid damage to cationisitis, the regeneration of acid and wash from the regeneration products is carried out without stopping, not allowing the gap between operations.
Before carrying out each subsequent cycle, the ion is shyling the flow of water from the bottom up so that all the ionship grains are in motion.
4.2.2. Through iones in the column, the regenerating solution is passed, the volume of which () in cubic centimeters is calculated by the formula
where is a given value of the specific flow rate of the regenerating agent, g / mol (g / g · eq);
- dynamic exchange tank; Choose according to the regulatory and technical documentation on a specific ionis, mol / m (r · eq / m); For ionites, AB-17-8, AN-31 and EDE-10P grades are allowed for the first regeneration increased value of dynamic exchange capacity to 3;
- Samples of Ionita, cm;
- concentration of regenerating mortar, G / DM.
The amount of regenerating solution is measured at the output of the column with a cylinder or benzur. Then the column is disconnected, the level of the solution over ion in the column is lowered to 1-2 cm and closed the lower for
4.2.3. The ionites after regeneration are washed with distilled water from an excess of acid (sodium hydroxide) at the rate indicated in Table 2.
Periodically select the filtrate sample and titrates sodium hydroxide solutions (acid) concentration (NaOH, HCl, HSO) \u003d 0.1 mol / dm (0.1 n.) In the presence of methyl orange (phenolphthalene).
The wash is controlled by table 2.
4.2.4. After washing, the column is filled with the impeller and set the saturation rate from Table 2.
When working in a column of working solutions of concentrations 0.01 mol / dm (0.01 n.) The filtrate is collected into a cylinder with a capacity of 250 cm, at a concentration of 0.0035 mol / dm (0.0035 n.) Use cylinder with a capacity of 1000 cm. The second and subsequent saturation cycles before the appearance of the working mortar ions in the filtrate (determined after the first cycle), the filtrate is collected by 100 and 250 cm, respectively, concentrations of the working solution.
4.2.5. To control saturation from portion of the filtrate, the sample is taken and analyzed in accordance with Table 2. If the analysis result shows that the saturation level did not reach the values \u200b\u200bspecified in Table 2, all previous filtrate samples can be not analyzed.
4.2.6. After appearing in the portion of the filtrate of the working solution ions in the amounts referred to in Table 2, the saturation is complete and calculated the total filtrate () and dynamic exchange container.
4.2.7. The ion is subjected to second regeneration and laundered in accordance with paragraphs.4.2.2 and 4.2.3.
When calculating the regenerating agent required for the second cycle, the value of the dynamic exchange container obtained in the first cycle in accordance with clause 4.2.6 is used in accordance with clause 4.2.6.
Before carrying out subsequent saturation cycles, the regenerating consumption consumption is calculated by the magnitude of the dynamic exchange capacity obtained in the previous cycle.
4.2.8. The definition end if the last cycles obtained the results allowed by the discrepancies between which do not exceed 5% of the average result, with the actual specific flow rate of the regenerating agent, differing from the specified norm by no more than 5%.
5. Processing results
5.1. Dynamic exchange capacity () in a cubic meter (r · eq / m) until the operating solution ions appear in the filtrate are calculated by the formula
where - the total volume of the filtrate passed through iones before the appearance of the ions of the working solution, see;
- the volume of ionet, see
5.2. The actual flow rate of the regenerating agent () in grams per mol (g / g of eq) of absorbed ions is calculated by the formula
where is the volume of regenerating mortar, see;
- concentration of regenerating solution, g / dm;
- the total amount of filtrate passed through iones until the ions of the working solution appears, see;
- Concentration of working solution, mol / dm (n.
5.3. Complete dynamic exchange capacity () in moles per cubic meter (r · eq / m) are calculated by the formula
where - the total amount of filtrate passed through iones to equalizing the concentrations of the filtrate and the working solution, cm;
- concentration of working solution, mol / dm (n.);
- the volume of portion of the filtrate after the appearance of the ions of the working solution (skip), cm;
- concentration of the solution in portions of the filtrate after the appearance of the ions of the working solution (skip), mol / dm (n.);
- ionet volume,
5.4. For the result of the definition, the average arithmetic results of the two latter cycles are taken, the discrepancies between which do not exceed ± 5%, with a trusted probability \u003d 0.95.
Note. When expressing the dynamic exchange capacity of ionites in a cubic meter under the word "mole", there is a molar mass of ion equivalent (Na, K, Ca, Mg, CL, NO, NSO, HSO, CO, SO, etc.).
The text of the document is drilled by:
official edition
Ionites. Methods of determination
exchange Capacity: Sat. Gostov. -
M.: IPK Publishing standards, 2002
General concepts
In general words, under the capacity of the ion exchange resin it is understood as the number of ions, which can be absorbed by a certain amount of resin. Moreover, the unit of measurement of the resin capacitance can be different. For example, MM-eq / ml (MEQ / ML), Mr. (EQ / L) or kilogran on cubic foot (KGR / FT3). Knowing an equivalent mass of matter, you can calculate the tank of the resin. The equivalent mass of the substance is defined as the ratio of the molar mass of the substance to its valence (strictly speaking, to the number of equivalence of the substance). For example, calcium molar weight is 40 g. / Mol, and valence 2, then the equivalent mass is equal to 20 g. / Mol (40/2 \u003d 20). The ion exchange resin with the exchange capacity of 1.95 g - eq / l is able to remove 1.95 h 20 \u003d 39 grams from the solution per 1 liter of resin.
In practice, the exchange capacity of the resin is determined in the titration laboratories. Through the column in which the soda is placed in a hydrogen form (H-form) is placed, sodium hydroxide solution (NaOH) is passed. Part of Na + ions is exchanged for hydrogen ions. Sodium hydroxide, which did not enter into a reaction with an ionic resin group, is cleaned with acid. Sut out from the initial concentration of sodium hydroxide, the residual concentration can be determined by the cation container. Another way to determine the exchange capacity of the ion is in passing through the resin layer of the calcium chloride solution. Similarly, the tank of an anion exchange resin (in OH-form) is determined, through which the acid solution is passed.
The resin capacitance can be measured in mM-eq / ml (volume) or MG-eq / g (weight). If a container expressed in mM-eq / g is defined (and the mass of dry ion is meant), then, knowing the humidity of the resin, it is easy to go to mM-eq / ml.
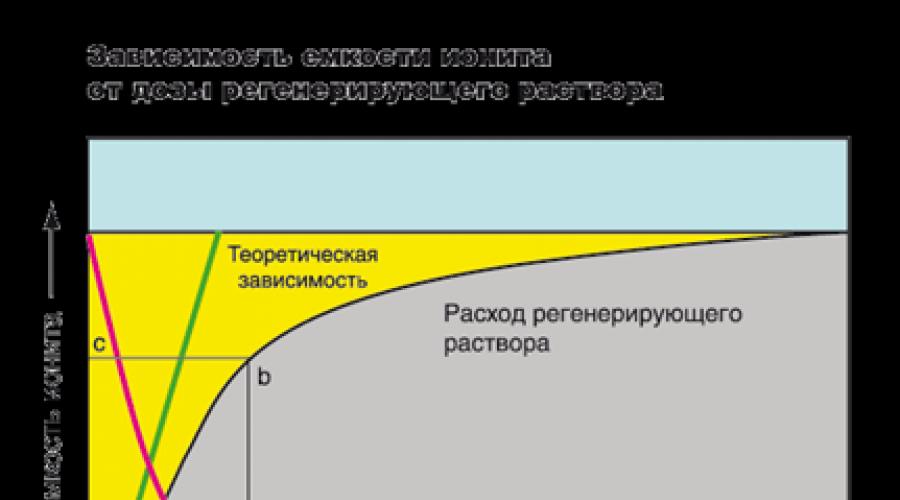
In the figure, the exchange capacity of the resin is graphically depicted by an area of \u200b\u200byellow color located between vertical straight and CL. The area of \u200b\u200bgray, located below the curve, is the concentration of ions in purified water. At the beginning of the cycle, the concentration of ions in the filtrate is very small, and remains constant throughout the filtercycle, at the moment when the filtering front reaches the end of the ione layer, the iones are occurring to the filtrate (in the figure - point P). This is a signal to resin regeneration. Usually, the filter regeneration is carried out to the slippath. For example, in industry, the concentration of rigidity ions in which the filter is derived to regeneration can reach a value of less than 0.05 03, and in domestic softening systems - less than 0.5 0. X - y segment length corresponds to the volume of purified water in liters or gallons. AnLB figure area is a complete absorption of the ions of the resin, and the Anmb figure area is the number of absorbed ions until the occurrence of the slippath.
Speaking about the tank, we often mean working precisely, and not a complete exchange container. Working capacity is not a permanent value, it depends on the set of factors: the brand of ionite, the concentration and type of the absorbed ions, the pH of the solution, on the requirements of the purified water, the flow rate, the height of the ionite layer and other requirements.

The achievement of a high degree of extraction of ions from an aqueous solution requires an increase in the dose of the regenerating solution (red line). However, increasing the concentration of the regenerating solution is infinitely impossible (the green line is the theoretical dependence between the degree of reduction of the resin capacitance and the flow rate of the regenerating solution). In practice, to achieve high capacity, it is necessary to increase the number of resins. With the first filtercycle, the degree of reduction of ion exchange properties can reach 100%, but over time this value will decrease. For example. Most of the manufacturers of water softening systems are recommended to use a NaCl solution with a concentration of 100-125 g. / l to restore the capacity of the cation of up to 50 - 55% of the total exchange container.
When determining the container, it is necessary to know the ionic form of the resin (salt, acid, main). With regeneration or in the process of operation, the volume of the pumped resin changes, the process called the resin called "breathing". The table shows how the resins behave in various processes.
Distinguish cation and anionics. Reactions in which iones are involved in the table.
ion exchange resin reaction titration

Moreover, in the English-language literature, the SAC symbol indicates the strongly acidic cation, SBA is a highly useful anion, WAC is a weakly acidic cation, and the WBA is a weakly-friendly anion. The ability to ion exchange is determined by the presence of a functional group, strongly acidic cations contain a sulfo group - SO3H, and the weakly acid-acid cation of the carboxyl group - COOH. The strongly acidic cations are exchanged by cations in any pH values, that is, they behave like strong acids in solution. A weakly acid-acid cation is similar to weak acids and react ion exchange only at pH values \u200b\u200babove 7. Anionics contain functional groups of five types: (-NH2, NH \u003d, N?, - N (CH3) 3OH, - N (CH3) 2OH4OH) . The first three groups give the anion of the poorly-friendly properties, and the groups - N (CH3) 3OH, - n (CH3) 2C2H4OH - strongly mining. The weakly-friendly anions react with anions of strong acids (SO, CL-, NO), and highly binding with anions of strong and weak (HCO, HSIO) in the pH range from 1 to 14. Speaking about the tank of highly binding anion, you should pay attention to That in the resin there are functional groups inherent in and poorly-friendly anionics. When aging highly binding anion or under the action of high temperatures, there is a decrease in baseline and partial destruction of functional groups.
Consider more reactions leaking with the participation of ion exchange resins. Reaction 1 - water softening on the strongly acidic cation of salt (Na) form, 2 - removal of nitrate ions on highly binding anion in CL-form. Application as a regenerating solution of sodium chloride and potassium chloride contributes to the widespread use of this type of resin in everyday life, industry and wastewater treatment. Cationias can also be restored by acid solutions (for example, hydrochloric acid), and anions - a solution of caustic soda (NaOH). The ionites in H and OH-form are used in the schemes for the preparation of desalted water (reaction 3 and 4). The weakly acidic cation is indicated by ion exchange properties at high pH values \u200b\u200b(reaction 5), and a weakly-axis anionite - at low pH values \u200b\u200b(reaction 6). Reaction 5 is simultaneous softening and decreased by alkalicity of water. It should be noted that WBA resin as a result of the regeneration with an alkaline solution is not transmitted in an OH form, but the so-called FB form (free base).
Weakly acidic cationis compared to the strong acids have a higher exchange capacity, they are characterized by a large affinity for hydrogen ions, so regeneration proceeds easier and faster. It is important that for the Regeneration of WAC, as well as WBA, the solutions of sodium chloride or potassium are not used. The choice of one or another brand of ion exchange resin depends on many conditions. For example, two types of highly mining anionics are distinguished: Type I (functional group - N (CH3) 3OH) and type II (-N (CH3) 2C2H4OH). The anions of type I are better absorb HSIO ions, in contrast to type II anionics, but the latter are characterized by a higher exchange capacity and better regenerated.
In conclusion, we note that in the literature, as well as in the passport on the products, the total weight and exchange tank of the resin is indicated, which are defined in the laboratory. The working capacity of the resin is lower than the manufacturer declared and depends on many factors that cannot be taken into account in the laboratory conditions (geometric characteristics of the resin layer, specific process conditions: streaming velocities, concentration of dissolved substances, degree of regeneration, etc.).
The materials of the Vion are used to clean the ventilation waste gas emissions from the soluble components, the aerosols of acids and salts of heavy metals, where they are used mainly in the form of non-woven needle climbing canvases.
Progress:
Weighed 2 gr. Kationita VION KN-1 (dry). Pour into the burest. Move the original CUCl 2 (3.6 mmol / l) through the column filled with cationite. Next, we grind the sample for 50 ml titration. Based on the methodology (paragraph 3.1), we determine the optical density of the sample and find the concentration of copper. The results are presented in Table 3.5.
Table 3.5
|
C, mmol / l |
||||
They built a graph of the dependence of the concentration of copper in the filtrate from the volume of the solution passed through the ionet.
Fig. 3.4.
The sorption process is to fully absorb first portions of cations by cation, and the absorption area is gradually moving along the column to the output. After that, the moment occurs when, due to the exhaustion of the cation container, the cations begin to get out of the column. From the graph, it can be seen that the concentration of copper at the outlet of the column increases gradually and has the form of the S-shaped curve, ranging from zero concentrations to the maximum. This curve is stretched with small concentrations of salts.
Calculated the amount of copper, which absorbed column to the total saturation of the cation, as the area of \u200b\u200bthe figure, a limited S-shaped curve and a direct maximum concentration:
h \u003d? VI * (Cmax - Ci) (3)
where Vi \u003d 50 ml,
Cmax \u003d 3,6mmmol
h1 \u003d 2.20 mmol.
Calculated the volumetric container of cation:
z1 \u003d H1 / M K \u003d 2.20 / 2 \u003d 1.10 mmol / gr. Kationata.
The discussion of the results
During the experimental work, the total exchange capacity of three different cations (KU-2-8, KU-1, VION KN-1) was determined. The results are presented in Figure 3.5.

The total exchange tank of the cation is proportional to the area of \u200b\u200bthe figure, a limited S-shaped curve and a direct maximum concentration. As can be seen from Figure 3.5. The containers of various ionites are different and less than the complete exchange capacity of the cations of the stated in the passport. So the complete exchange capacity of KU-2-8 cation is found experimentally below the passport value by 28%, the complete exchange capacity of KU-1 is lower than the passport value by 57%, and the cation of the VION KN-1 is 39%. These data must be taken into account when calculating and designing ion exchange apparatuses and filters.
Ion exchange proceeds on those adsorbents that are polyelectrolytes (ion exchangers, ionites, ion exchange resins).
Ion exchangethe equivalent ionization exchange process in the ion exchanger is called other ions of the same sign in solution. The process of ion exchange is reversible.
Ionites are divided into cations, aniones and amphoteric ionites.
Kationites- Substances containing fixed negatively charged groups in their structure (fixed ions), which are moving moving cations (counterions), which can exchange with cations in the solution (Fig. 81).
Fig. 81. Model of a polyelectrolyte matrix (cation) with fixed anions and moving counterions, where - fixed ions;
- COIONES, - Councilions
Natural cations: zeolites, permutitis, silica gel, cellulose, as well as artificial: high molecular weight solid insoluble ionic polymers containing most often sulfo groups, carboxyl, phosphine-acid, arsenic acid or selenium groups. Synthetic inorganic cations, which are most often used by aluminosilicates.
According to the degree of ionization of ionic groups, the cations are divided into severe acid and weakness. The strongly acidic cations are capable of exchanging their mobile cations on external cations in alkaline, neutral and acidic environments. Weakly acid cation exchanges exchange counterions on other cations only in an alkaline environment. The strongly acidic are cationis with strongly dissociated acid groups - sulfonic acid. Weakly acid include cationis containing weakly acidic acid groups - phosphorous acid, carboxyl, oxyphenyl.
Anionites- ion exchangers that contain positively charged ionic groups in their structure (fixed ions), which are moving anions (counterions), which can exchange with anions that are in solution (Fig. 82). Distinguish natural and synthetic anionics.

Fig. 82. Model of the polyelectrolyte matrix (anion) with fixed cations and moving counterions, where + are fixed ions;
- COIONES, - Councilions
Synthetic anionics contain positively charged ionic groups in macromolecules. Weakly-homely anionics are in their composition primary, secondary and tertiary amino groups, highly binding anions contain groups of quaternary onion salts and bases (ammonium, pyridinium, sulfonium, phosphonium). Highly binding anions exchange moving anions in acidic, neutral and alkaline media, weakly-axis - only in an acidic environment.
Amphoteric Ionitescontain both cationic, and anionic ionic groups. These iones can sorbites both cations and anions.
The quantitative characteristic of ion is full exchange tank(PO). The determination can be carried out by a static or dynamic method based on reactions flowing in the Ionbit - Solution system:
RSO 3 - H + + NaOH → RSO 3 - Na + + H 2 O
RNH 3 + OH - + HCl → RNH 3 + Cl - + H 2 O
The capacity is determined by the number of ionic groups in ionate and therefore theoretically there must be a constant value. However, it practically depends on a number of conditions. There are static exchange tanks (SEO) and dynamic exchange capacity (DEE). Static exchange capacity is a complete container that characterizes the total number of ionic groups (in milliecquivalents) per unit mass of air-dry ion or NA unit of the amount of swollen ionis. Natural ionites have a small static metabolic capacity, not exceeding 0.2-0.3 MEKV / g. For synthetic ion exchange resins, it is within 3-5 MEKV / g, and sometimes reaches 10.0 meq / g.
Dynamic, or working, exchange capacity applies only to the part of the iongepps, which are involved in the ion exchange that occurs in technological conditions, for example, in an ion exchange column at a certain relative speed of the ionite and solution. The dynamic capacity depends on the speed of movement, the size of the column and other factors and is always less than the static metabolic capacity.
To determine the static exchange tank of ionites, various methods are used. All these methods are reduced to the saturation of ionet by any ion, then displacing it with another ion and the analysis of the first in solution. For example, the cationite is conveniently translated into H + -form (the counterions are hydrogen ions), then rinse with sodium chloride solution and the resulting sour solution is removed with alkali solution. The capacity is equal to the ratio of the amount of acid that has passed into a solution of an acid to the mood of ionon.
With a static method, the acid or alkali is titrated, which as a result of ion exchange adsorption appear in solution.
At a dynamic method, this is determined by chromatographic columns. Through a column filled with ion exchange resin, the electrolyte solution is passed and the dependence of the concentration of the absorbed ion in the outdated solution (eluate) is recorded from the volume of the past solution (output curve). Pose calculated by the formula
 , ,
| (337) |
where V. total - the total volume comprising an acid supplied from resin; from - a concentration of acid in this solution; m. - Mass of ion exchange resin in the column.
The equilibrium exchange constant can be determined from data on the equilibrium distribution of ions in static conditions (an equilibrium state of ion exchange is described by the law of the mass), as well as the dynamic method for the rate of moving the zone of the substance along the resin layer (eluent chromatography).
For the reaction of ion exchange
![]()
equilibrium constant is equal
 , ,
| (338) |
where, - the concentration of ions in ionet; - The concentration of ions in solution.
Applying ionics, you can soften water or desalinate saline water and getting suitable for pharmaceutical purposes. Another use of ion exchange adsorption in pharmacy is to use it for analytical purposes as a method for extracting from the mixtures of one or another component being analyzed.
Examples of solving problems
1. In 60 ml of a solution with a concentration of some substance, 0.440 mol / l placed activated carbon weighing 3 g. The solution with an adsorbent was shaken to establish an adsorption equilibrium, as a result of which the concentration of the substance decreased to 0.350 mol / l. Calculate the adsorption value and the degree of adsorption.
Decision:
Adsorption is calculated by formula (325):

By formula (326), determine the degree of adsorption
2. According to the given data, for the adsorption of DIMEDROL on the surface of the coal, calculate graphically constants of the Langmur equation:
Calculate the adsorption of DIMEDROL at a concentration of 3.8 mol / l.
Decision:
To graphically determine the constants of the Langmuir equation, we use the linear shape of this equation (327):
Calculate 1 / but and 1 / from:
Build a schedule in coordinates 1 / but – 1/from(Fig. 83).
Fig. 83. Graphic definition of the constants of the Langmur equation
In the case when the point h.\u003d 0 is located outside the drawing, use second way y \u003d AX + B. First, choose two any points lying on the straight (Fig. 83) and determine their coordinates:
(·) 1 (0.15; 1,11); (·) 2 (0.30; 1.25).

b \u003d Y 1 - AX 1 \u003d0.11 - 0.93 · 0.15 \u003d 0.029.
We get that b. = 1/but ¥ \u003d 0.029 μmol / m 2, therefore but ¥ \u003d 34.48 μmol / m 2.
Constant adsorption equilibrium K.determined as follows:
Calculate the adsorption of diphrolol at a concentration of 3.8 mol / l by the Langmuir equation (327):
3. When studying adsorption of benzoic acid on a solid adsorbent, the following data obtained:
Decision:
To calculate the constants of the Freundlich equation, it is necessary to use the linear form of equation (332), in the coordinates of LG ( x / T.) – lG. from Isothermary has the kind of direct.
Find LG values c. and LG. x / M.included in the Linearized Freundlich Equation.

| LG. c. | –2,22 | –1,6 | –1,275 | –0,928 |
| LG. x / M. | –0,356 | –0,11 | 0,017 | 0,158 |
Build a schedule in the coordinates of LG ( x / T.) – lG. from(Fig. 84) .
Fig. 84. Graphic definition of the constants of the Freundlich equation
Since point h.\u003d 0 located outside of the picture (84), we use second way Definition of coefficients direct y \u003d AX + B(See "Introductory block. Fundamentals of mathematical processing of experimental data"). First, choose two any points lying on a straight line (for example, points 1 and 2) and determine their coordinates:
(·) 1 (-2.0; -0.28); (·) 2 (-1.0; 0.14).
Then we calculate the angular coefficient by the formula:

b \u003d Y. 1 - AX. 1 = -0.28 - 0.42 · (-2.0) \u003d 0.56.
The constants of the Freundlich equation are equal:
lG. K \u003d b \u003d0,56; K.= 10 0,56 = 3,63;
1/n \u003d a \u003d0,42.
Calculate the adsorption of benzoic acid at a concentration of 0.028 mol / l using the Freundlich equation (330):
4. Using the BET equation, calculate the specific surface of the adsorbent according to the data on the adsorption of gaseous nitrogen:
The area occupied by a nitrogen molecule in a dense monolayer is 0.08 nm 2, a nitrogen density is 1.25 kg / m 3.
Decision:
Equation of polymolecular adsorption of BET in linear form has the form (333)
To build a graph, determine the values:
Build a schedule in coordinates - P / P S (Fig. 85).
Using first method (See "Introductory block. Basics of mathematical processing of experimental data") Definition of direct coefficients y \u003d ax + b. We define the value of the coefficient b.as the ordinate of the point lying on a direct, which is the abscissa equal to 0 ( h.= 0): b. \u003d 5. Select the point on the straight and determine its coordinates:
(·) 1 (0.2; 309).
Then we calculate the angular coefficient:


Fig. 85. Graphic definition of the constants of the polymolecular adsorption of BET isotherm
Constants Equation of isotherm of polymolecular adsorption BET are equal:
 ; .
; .
Solving the system of equations, get but ∞ \u003d 6.6 · 10 -8 m 3 / kg.
To calculate the limit value of adsorption, we will take but ∞ to 1 mol:
 .
.
The value of the specific surface of the adsorbent is found by formula (329):
5. Polystyrene sulfocathionitis in N + -form Weight 1 g were introduced into a solution of KCl with a starting concentration from 0 \u003d 100 eq / m 3 volume V.\u003d 50 ml and the mixture was kept to an equilibrium state. Calculate the equilibrium concentration of potassium in ionate, if the constant of the ion exchange equilibrium \u003d 2.5, and the total exchange tank of the cation is PO \u003d 5 mol-eq / kg.
Decision:
To determine the constant of ion exchange, use equation (338). In resin, H + ions exchange on the equivalent number of ions K.
The mass of sulfocathionitis in H +-form is determined by the formula (337):


The total number of anion in it --form is equal to:
The mass of anion in it is also determined by the formula (337):
