Human body heat exchange with the environment. Heat Pot.
IN. BUT. Vinogradov- Saltykov, National university food technology (g.. Kiev), IN. G.. Fedorov, Open international university development man "Ukraine" (g.. Kiev), IN. P. Marznce., Branch Kyivenergo "Zhilteterenergo" (g.. Kiev)
It is shown that the actual heat loss from the outer surfaces of the water boilers Q 5 is significantly less than the regulatory losses, which were determined by schedules or tables compiled for steam boilers of high performance by extrapolation into the area of \u200b\u200blow thermal productivity of boilers. Such a decrease in Q 5 is explained by lower temperatures of the outer surfaces. So, when transferring the DCVR steam boiler to the water treatment, the temperature modes of all elements of the boiler occurs, which leads to a decrease in the loss of heat into the environment.
To determine Q 5, direct measurements of the density of the heat flux q from the outer surfaces of the boiler using small minority heat meters are carried out. The distribution of heat loss in separate surfaces of steam and water boilers was uneven, therefore, for calculating Q 5, local values \u200b\u200bof q were measured within each surface, combining the gradient method for searching the maximum thermal loss and the scanning method, as well as using statistical methods to averaging experienced data on the surface and time.
Averaging Thus, the value Q (W / m 2) for each element f (m 2) of the outer surface of the boiler was used to calculate Q 5:
where QHR is the lowest heat combustion of gas to the working mass, J / M 3; B - Gas consumption, m 3 / s.
The experiments were carried out, as a rule, in the conditions of production of boilers, i.e. Their productivity differed from the nominal. Therefore, the inverse dependence of thermal losses from the actual heating capacity of the boiler was accepted for steam boilers.
where D and Q 5 is the actual capacity of the boiler and heat loss from the outer surfaces, D h and q 5 h is the same for nominal conditions.
For checking (2), there were experiments on the KPG-6.5 boiler, the front and side walls of which, after disassembling the brick surroundings, were replaced with chamoten-fibrous plates of SPGT-450. To change the heat capacity of the boiler, the gas flow rate was changed and, accordingly, the increase in water temperature in the boiler, maintaining water consumption permanent. In the range of D change, the maximum possible for the operating conditions of the boiler, formula (2) was fair: recalculation for it for all actual D gave the almost the same value Q 5 H \u003d 0.185%. For the CFG-6.5 boiler with the traditional dressing of the test showed the loss of heat Q 5 H \u003d 0.252%. With a complete replacement of climbing on the SPGT-450 plate and a thorough seal of joints between them, it is possible to calculate the decrease in Q 5 and the gas consumption by 0.10-0.15%. With a mass replacement of irrigation during repairs, this may make a significant contribution to energy and resource saving, since the reduction of gas consumption by 0.1% in the system of the KievEnergo branch of "Zhiltetelloener" leads to a gas savings of 1300 m s / day. .
Conclusions were confirmed from the fact that the actual heat losses from the outer surfaces of the water boilers several times lower than the regulatory. Thus, the developers of compact boilers TWG, the staff of the Institute of Gas National Academy of Sciences of Ukraine, when conducting acceptance tests, measured surface thermometers  the average temperature of the outer surfaces of the walls of the boilers and according to the known formulas was calculated by Q 5. For boilers TVG-4 and TVG-8, regulatory losses are 2%, and the calculated increased by reducing the load from the nominal to the minimally appropriate for TVG-4 from 0.54 to 1%, for TVG-8 from 0.33 to 0.94 %. Therefore, the Institute recommended in 2000 organizations operating boilers of this type, to take the average value Q 5 \u003d 0.75%.
the average temperature of the outer surfaces of the walls of the boilers and according to the known formulas was calculated by Q 5. For boilers TVG-4 and TVG-8, regulatory losses are 2%, and the calculated increased by reducing the load from the nominal to the minimally appropriate for TVG-4 from 0.54 to 1%, for TVG-8 from 0.33 to 0.94 %. Therefore, the Institute recommended in 2000 organizations operating boilers of this type, to take the average value Q 5 \u003d 0.75%.
Such conclusions came to the study of KVG boilers developed at the Institute of Gas National Academy of Sciences of Ukraine. To determine Q 5, the formula (1) was also used here, but instead of 2 (CJF) substituted QF k, where F K is the total outer area of \u200b\u200bthe thermal insulation of the boiler. The average Q value was calculated by the formula:
Here, the density of the heat flux from the outer surface of insulation to the air Q o and from the inner surface to the air Q T is determined from the formulas:
![]()
where A is the total heat transfer coefficient to the environment; t 0, t t, t b - the temperature of the outer, inner surface and air; R is the total thermal resistance of layers of irrigation; R 0 \u003d 1 / a 0.
T T T and T 0 values \u200b\u200bare recommended to be determined by direct measurements or calculated method, R - to calculate depending on the thickness and thermal conductivity of the insulation layers, and a 0 - according to the known formulas of the cammerler for flat and cylindrical surfaces.
When calculating Q 0 and q t, their values \u200b\u200bdiffered significantly, although in the stationary operation of the boiler they are almost the same. The reason that Q T\u003e Q 0 was obtained by the fact that due to the inevitable forced circulation of air in the room of the boiler room, the actual values \u200b\u200bof A 0 by 12-15% more calculated, as was shown by direct measurements Q 0 and (T 0 - T B on a steam boiler TGMP-314A. Because of this difference in Q 0 and Q T in (3), it was introduced to - the correction coefficient of measurement error and calculations Q 0 and Q T, which is recommended to take from 0.3-0 , 7. Apparently, with the same confidence in both values, it is necessary to take them to half a half.
For accounting for additional heat loss through thermal bridges, the coefficient to m \u003d 0.2-0.4 is introduced.
In addition to introducing K and K M, it is proposed to increase Q 5 by 10-20% to account for heat losses through the lower (sub-case) hard-to-reach surface of the boiler, as well as take into account the share of the loss from the outer surfaces, which returns to the firebox and gas supplies of the boiler along with air From boiler room.
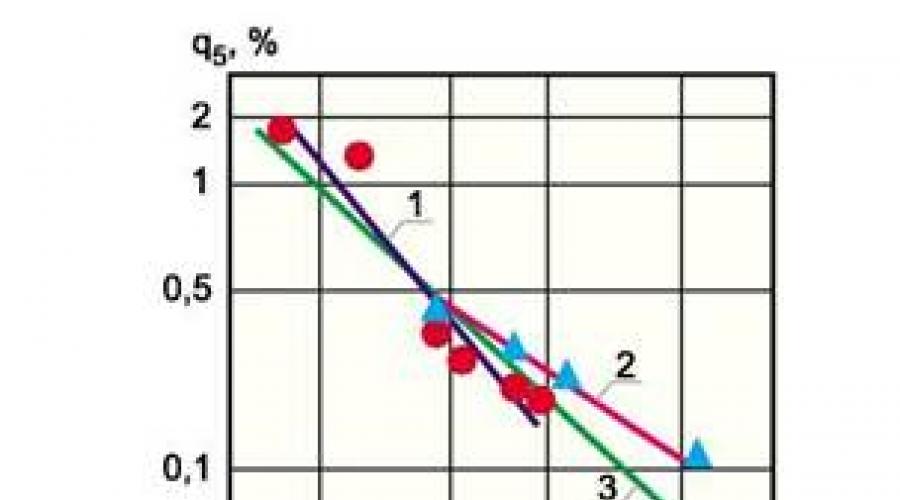
Despite the significant differences in the method of determining the Q 5 V and, the results turned out to be similar, which gives grounds for generalizing these results and their use in the preparation of regulatory documents. The figure shows the dependence Q 5 of the nominal thermal production capacity of the water-heating boilers NIISU-5, NIIS-5X2, TVG-4, TVG-8, KVG-4, KVG-6.5, as well as the COG-4, KVG-6,5, KVGM -10 and KVGM-50. Data from and is somewhat lower than the relevant data from, however, such a difference is fully justified by different methods of research.
Literature
1. Fedorov IN. G.., Vinogradov- Saltykov IN. BUT., Marznce. IN. P. Measure lOSS heat from outdoor surfaces water Heat boilers // Ecotechnologies and resource saving. 1997. № 3. FROM. 66-68.
2. Marznce. IN. P., Fedorov IN. G.. Efficiency insulating fences water Heat boilers // Prom.. heat engineering. 2000. T.. 22, № 2. FROM. 78-80.
3. Fedor I.in IN. G.., Vinogradov- Saltikov IN. BUT., Marznce. IN. P. Rippodei.l. teplothrat by gentlemen water I.yNI taparium boileri.in / Udoht. TO., 1998. 16 from. Dep. in Dntb UK- r.i.n.23.03.98, № 142.
4. Fedorov IN. G.., Pleskonos. BUT. TO. Planning and sales experiments in foodie industry. M..: Food. prom.- st, 1980. 240 from.
5. Magca. AND., Golyashev. IN., Mesaki. FROM. Methodik definitions losses heat vapor boiler in environment// Heat and power. 2001. № 10. FROM. 67-70.
6. Zalkind E.. M.. Materials cutting and payment fences steam boilers. M..: Energy, 1972. 184 from.
7. CammereRj.s. Erleuchtungen Zu Den VDI - Rechtlinien Fuerwaerme - Und Kalteschutz - Brennstoff - Waerme - Kraft.1958. BD.10, № 3. S.119-121.
8. Fedorov IN. G.., Vinogradov- Saltykov IN. BUT., Novik M.. AND. Heatherria outdoor surfaces boiler TGMP-314 BUT // Ecotechnologies and resource saving. 1999. № 4. FROM. 77-79.
For reducing the consumption of warmth need strict accounting for thermal losses in technological equipment and thermal networks. Thermal losses depend on the type of equipment and pipelines, the correct operation and the type of insulation.
Thermal losses (W) are calculated by the formula
Depending on the type of equipment and pipeline, total thermal resistance is:
for insulated pipeline with one layer of isolation:

for an insulated pipeline with two layers of isolation:

for technological apparatus with multilayer flat or cylindrical walls with a diameter of more than 2 m:
for technological devices with multilayer flat or cylindrical walls with a diameter of less than 2 m:

pitel to the inner wall of the pipeline or apparatus and from the outer surface of the wall to the environment, W / (m 2 - K); X TR ,?. Art, Xj is the thermal conductivity of the material of the pipeline, insulation, the walls of the apparatus, / native of the wall layer, W / (m. K); 5 tbsp. - wall thickness of the apparatus, m.

The heat transfer coefficient is determined by the formula

or by empirical equation
The transfer of heat from the walls of the pipeline or the machine to the environment is characterized by a coefficient A H [W / (M 2 K)], which is determined by criteria or empirical equations:
according to criteria equations:

The heat transfer coefficients A B and A H is calculated by criteria or empirical equations. If the hot heat carrier is hot water or condensing couples, then in\u003e and n, i.e. R b< R H , и величиной R B можно пренебречь. Если горячим теплоносителем является воздух или перегретый пар, то а в [Вт/(м 2 - К)] рассчитывают по критериальным уравнениям:
according to empirical equations:
Thermal insulation of devices and pipelines is made of materials with low thermal conductivity. Well-chosen thermal insulation reduces heat loss into the surrounding space by 70% or more. In addition, it increases the performance of heat plants, improves working conditions.
Thermal insulation of the pipeline consists mainly of one layer coated from above for the strength of a layer of sheet metal (roofing steel, aluminum, etc.), dry plasters from cement solutions, etc. In the case of using a coating layer of metal with thermal resistance, it is possible to neglect it. If the coating layer is plaster, its thermal conductivity is slightly different from the thermal conductivity of thermal insulation. In this case, the thickness of the cover layer is, mm: for pipes with a diameter of less than 100 mm - 10; for pipes with a diameter of 100-1000 mm - 15; For pipes with a large diameter - 20.
The thickness of the thermal insulation and the coating layer should not exceed the maximum thickness depending on the mass loads on the pipeline and its overall dimensions. In tab. 23 shows the values \u200b\u200bof the limiting thickness of the insulation of steam pipelines, recommended by the standards for the design of thermal insulation.
Thermal insulation of technological devices It may be single-layer or multi-layered. Warm loss through thermal

insulation depend on the type of material. The heat loss in pipelines is calculated on 1 and 100 m of pipeline length, in the technological equipment - 1 m 2 of the surface of the device.

The layer of contamination on the inner walls of pipelines creates an additional thermal resistance to the transfer of heat into the surrounding space. Thermal resistances R (m. K / W) when moving some coolants have the following values:

In pipelines that feed technological solutions to the devices and hot heat carriers to heat exchangers, there are shaped parts in which part of the heat of the flow is lost. Local light losses (W / m) are determined by the formula

The coefficients of local resistances of the shaped parts of pipelines have the following values:
When compiling Table. 24 The calculation of specific heat losses was carried out for steel seamless pipelines (pressure< 3,93 МПа). При расчете тепловых потерь исходили из следующих данных: тем-

the air peraculus in the room was taken equal to 20 ° C; Its speed with free convection - 0.2 m / s; steam pressure - 1x10 5 Pa; water temperature - 50 and 70 ° C; The thermal insulation is made in one layer of asbestos cord, \u003d 0.15 W / (m. K); The heat transfer coefficient A "\u003d 15 W / (m 2 - K).
Example 1. Calculation of specific thermal losses in the steam loss.


Example 2. Calculation of specific heat losses in an uninsulated pipeline.
Conditions
Pipeline with a steel diameter of 108 mm. The diameter of the conditional passage D y \u003d 100 mm. The temperature of the steam 110 ° C, the environment 18 ° C. Thermal conductivity of steel X \u003d 45 W / (m. K).
The obtained evidence suggests that the use of thermal insulation reduces thermal losses per 1 m pipeline length 2.2 times.
Specific thermal losses, W / m 2, in technological devices of leather and felt-felt production are:

Example 3. Calculation of specific thermal losses in technological devices.
1. The drum "Giant" is made of larch.


2. Dryer of the company "Khirac Kinzoku".

3. Barcas for dyeing takes. Made of stainless steel [K \u003d 17.5 W / (M-K)]; There is no heat insulation. The overall dimensions of the barcas 1.5 x 1.4 x 1.4 m. Wall thickness 8 st \u003d 4 mm. Process temperature T \u003d \u003d 90 ° C; Air in the workshop / cp \u003d 20 ° C. Air speed in the workshop V \u003d 0.2 m / s.

The heat transfer coefficient A may be calculated as follows: A \u003d 9.74 + 0.07 AT. With / cf \u003d 20 ° C A is 10-17 W / (m 2. K).
If the surface of the coolant of the device is open, specific thermal losses from this surface (W / m 2) are calculated by the formula
Industrial service "Caprikorn" (United Kingdom) proposes to use the Alplas system to reduce heat losses from the open surfaces of coolants. The system is based on the use of hollow polypropylene floating balls, almost completely covering the surface of the fluid. The experiments showed that at a water temperature in an open tank 90 ° C, thermal losses when using a layer of balls decrease by 69.5%, two layers - by 75.5%.
Example 4. Calculation of specific thermal losses through the walls of the drying unit.
The walls of the drying unit can be made of various materials. Consider the following wall designs:
1. Two layers have become a thickness of 5 st \u003d 3 mm with an insulation located between them in the form of an asbestos plate 5 and \u003d 3 cm and thermal conductivity x and \u003d 0.08 W / (m.).
Table of contents of the topic "Regulation of metabolism and energy. RATIONAL POWER. Main exchange. Temperature of the body and its regulation.":1. Energy costs of the body under exercise conditions. The coefficient of physical activity. Working boost.
2. Regulation of metabolism and energy. Center for the regulation of metabolism. Modulators.
3. Concentration of glucose in the blood. Glucose concentration regulation circuit. Hypoglycemia. Hypoglycemic coma. Hunger.
4. Nutrition. Nutrition. The ratio of proteins, fats and carbohydrates. Energy value. Calorie.
5. The diet of pregnant and lactating women. Baby diet. Distribution of daily diet. Alimentary fiber.
6. Rational power as a factor in conservation and health promotion. Healthy lifestyle. Food recovery mode.
7. Temperature of the body and its regulation. Homoothermal. Poikilotermic. Isothermia. Heterothermal organisms.
8. Normal body temperature. Homoothermal kernel. Poikilotermic shell. Comfort temperature. Person's body temperature.
9. Heat Production. Primary warmth. Endogenous thermoregulation. Secondary warmth. Contracting thermogenesis. Non-culture thermogenesis.
There are the following ways to recover the heat by the organism Environment: radiation, heat Conduction, convection and evaporation.
Radiation - This is a way of recoil heat into the environment of the human body surface in the form of electromagnetic waves of the infrared range (A \u003d 5-20 μM). The amount of heat dissipated by the body into the environment by radiation is proportional to the area of \u200b\u200bthe radiation surface and the difference in the average values \u200b\u200bof skin temperature and the environment. The area of \u200b\u200bthe radiation surface is the total surface area of \u200b\u200bthose parts of the body, which come into contact with air. At ambient temperature of 20 ° C and relative air humidity of 40-60%, the body of an adult dispels by radiation about 40-50% of the total heat. The heat transfer by radiation increases when the ambient temperature decreases and decreases when it is increased. In conditions of constant ambient temperature, radiation from the surface of the body increases with increasing skin temperature and decreases when it decreases. If the average temperature of the skin and the environment is aligned (the temperature difference becomes zero), heat returns to radiation becomes impossible. It is possible to reduce the heat transfer of the body by radiation by reducing the area of \u200b\u200bthe radiation surface ("boding the body in the ball"). If the ambient temperature exceeds the average skin temperature, the human body, absorbing infrared rays emitted by the surrounding objects is warmed.
Fig. 13.4. Types of heat transfer. The way the heat of heat into the external environment can be conventionally divided into "wet" heat transfer associated with the evaporation of sweat and moisture from the skin and mucous membranes, and on the "dry" heat transfer that is not associated with loss of fluid.Heat Conduction - A method of recoil heat, having a place when contacting the body contact with other physical bodies. The amount of heat given to the organism into the environment in this method is proportional to the difference in the average temperature of the contacting bodies, the area of \u200b\u200bcontacting surfaces, the heat of thermal contact and the thermal conductivity of the inactive body. Dry air, adipose tissue is characterized by low thermal conductivity and are thermal insulators. The use of fabrics containing a large number of small fixed "bubbles" of air between fibers (for example, woolen fabrics) gives the human body to reduce heat scattering by heat conduction. Wet, saturated with water vapor air, water is characterized by high thermal conductivity. Therefore, the residence of a person in a high humidity medium at low temperatures is accompanied by an increase in the body's heat loss. Wet clothing also loses its heat insulating properties.
Convection - The method of heat transfer of the body, carried out by transferring heat by moving particles of air (water). For heat scattering, convection requires strengthening the surface of the body with a lower temperature with a lower temperature than the skin temperature. At the same time, the air layer intensive with the skin is heated, reduces its density, rises and is replaced by colder and more dense air. Under conditions, when the air temperature is 20 ° C, and the relative humidity is 40-60%, the body of an adult dispels into the environment by heat-carrying and convection of about 25-30% of heat (basic convection). With increasing speed of air flow (wind, ventilation), the intensity of heat transfer (forced convection) is significantly increasing.
Revolution of heat by the organism way heat Conduction, convection and spontaneouscentered "Dry" heat transferbecomes ineffective when aligning the average temperature of the body and the environment.

Heat transfer by evaporation - This is the method of scattering the body of heat into the environment due to its costs of evaporation of sweat or moisture from the surface of the skin and moisture from the mucous membranes of the respiratory tract ("wet heat transfer). A person is constantly released by swelling of the skin of the skin ("tangible", or iron, water loss), the mucous membranes of the respiratory tract ("irrevomable" water loss) are moistened (Fig. 13.4). At the same time, the "tangible" water loss by the body has a more significant impact on the total amount of heat dissipated by evaporation than "irrelevant".
At a temperature of an external environment, about 20 "with evaporation of moisture is about 36 g / h. Since the evaporation of 1 g of water in humans is spent 0.58 kcal of thermal energy, it is not difficult to calculate that by evaporation of the adult body, it gives in these conditions to the environment 20% of the total heat dissipation. Increase the external temperature, physical work, long-term stay in thermal insulation clothing reinforce the sweating and it may increase to 500-20 g / h. If the external temperature exceeds the average skin temperature, then the body cannot be given to the external environment Heat radiation, convection and heat transfer. The body under these conditions begins to absorb heat from the outside, and the only way of heat scattering becomes an increase in moisture evaporation from the body of the body. Such evaporation is possible until the humidity of the ambient air remains less than 100%. With intensive sweating, high humidity and low air movement speed when ka PAT PAT, not soying to evaporate, merge and flow from the body surface, heat transfer by evaporation becomes less efficient.
The exchange of thermal energy between the organism and the environment is called heat exchange. One of the heat exchange rates - the body temperature, which depends on two factors: heat formation, that is, from the intensity of metabolic processes in the body, and the impact of heat into the environment.
Animals whose body temperature varies depending on the temperature of the external environment, are called poikilotermanor cold-blooded. Animals with constant body temperature are called homootherm (warm-blooded). Constancy of temperaturethe bodies are called isother miay.. She is provides independenceexchange processes in tissues and organs from temperature fluctuationsenvironment.
Person's body temperature.
The temperature of individual parts of the human body is different. The lowest temperature of the skin is marked at the brushes and footsteps, the highest - in the axillary depression, where it is usually determined. In a healthy persontemperature in this areas of equal 36-37 ° C. During the day, small lifts and decay of the human body temperature are observed in accordance with the daily biorhythm:the minimum temperature is marked in 2- 4 ch nights, the maximum - at 16-19 hours.
T. empress muscular fabric B. the state of rest and work can fluctuate in the range of 7 ° C. The temperature of the internal organs depends from exchange intensity processes. The most intense exchange processes proceed in the liver, which is the hottest organ of the body: The temperature in the liver tissues is 38-38.5 ° FROM. The temperature in the rectum is 37-37.5 ° C. However, it can vary in the range of 4-5 ° C, depending on the presence of hiding masses, the blood flow of its mucous and other reasons. Runners on large (marathon) distances at the end of the competition temperatures in the rectum can increase to 39-40 ° C.
The ability to maintain the temperature at a constant level is ensured by interrelated processes - heat formation and Heat release From the body into an external environment. If the heat generation is equal to the heat transfer, then the body temperature remains constant. The process of formation of heat in the body got a name chemical thermostat, process that ensures the removal of heat from the body, - physical thermoregulation.
Chemical thermoregulation. The thermal exchange in the body is closely related to the energy. When oxidizing organic substances, energy is released. Part of the energy goes to the synthesis of ATP. This potential energy can be used by the body in its future activities.The source of heat in the body is all tissues. Blood, flowing through the fabric, heats up.
An increase in ambient temperature causes a reflex reduction in metabolism, as a result of this, heat generation decreases in the body. With a decrease in the ambient temperature, the intensity of metabolic processes increases reflectically and heat generation is enhanced. To a greater degree, the increase in heat generation occurs due to an increase in muscle activity. Incoming muscle contractions (tremor) are the main form of heat generation. The increase in heat generation can occur in muscle tissue and due to the reflex increase in the intensity of metabolic processes - non-conscientive muscular thermogenesis.
Physical thermoregulation. This process is carried out by recovering heat into the external environment by convection (heat transfer), radiation (heat emission) and water evaporation.
Convection - The immediate return of heat adjacent to the skin subjects or particles of the medium. The return of heat is more intense than the difference between the temperature between the body and the ambient air.
The heat transfer increases when air movement, for example, with wind. The intensity of heat recovery largely depends on the thermal conductivity of the environment. In water, heat returns is faster than in air. Clothing reduces or even stops heat-country.
Radiation - the separation of heat from the body occurs by infrared radiation from the body surface. Due to this, the body loses the bulk of the heat. The intensity of heat control and heat emission is largely determined by the skin temperature. Heat transfer regulates the reflex change in the lumen of the skin vessels. With increasing ambient temperature, arterioles and capillaries occur, the skin becomes warm and red. This increases heat transfer and heat emission processes. When the air temperature decreases, the arteriole and skin capillaries are narrowed. The skin becomes pale, the amount of blood flowing through its vessels decreases. This leads to a decrease in its temperature, the heat transfer is reduced, and the body maintains heat.
Evaporation of water From the body surface (2 / s moisture), as well as in the process of breathing (1 / s moisture). Evaporation of water from the body surface occurs when the sweat is selected. Even with the complete absence of visible sweating through the skin evaporates per day up to 0.5 L. Waters - invisible sweating. Evaporation of 1 l sweat in a person with a body weight of 75 kg can reduce body temperature at 10 ° C.
In the state of relative rest, an adult shall highlight 15% heat into the external medium by heat-resignation, about 66% by heat emission and 19% by evaporation of water.
On average, man loses per dayabout 0.8. l sweat, and with it 500 kcal heat.
With the breath of man alsoit highlights about 0.5 liters of water.
At low ambient temperature ( 15 ° C and below) About 90% of the daily heat transfer occurs due to heat transfer and heat emission. Under these conditions, there is no visible sweating.
At air temperature 18-22 ° With heat transfer due to thermal conductivity and heat emission decreases, butincreases lossheat by organism by evaporationmoisture from the skin surface. With high humidity, when water evaporation is difficult, overheating may occurbody and developthermal hit.
Light-Permeable Water Vapor clothes prevents effective sweating andmay be the cause human organism overheating.
In hot countries, with long campaigns, in hot workshops man loses a large number fluids from then. At the same time there is a feeling thirst that is not quenched water. it associated with that S. then a large amount of mineral salts is lost. If add to drinking water salt, that feeling thirst disappeared and the well-being of people will improve.
Heat transfer regulation centers.
The thermoregulation is carried out reflexively. Environmental fluctuations are perceived thermoreceptor. In large quantities, thermistors are located in the skin, in the mucous membrane of the oral cavity, the upper respiratory tract. Thermoreceptors in the internal organs, veins, as well as in some formations of the central nervous system, are detected.
Skin thermistors are very sensitive to ambient temperature fluctuations. They are excited by increasing the temperature of the medium by 0.007 ° C and the decrease - by 0.012 ° C.
Nervous impulses arising in thermistors, according to afferent nerve fibers come in the spinal cord. According to conducting paths, they reach visual bugs, and they go to the hypothalamic region and to the core of a large brain. As a result, there is a feeling of warmth or cold.
In the spinal curtain There are centers of some temperature regulatory reflexes. Hypothalamus It is the main reflex center of thermoregulation. The front hypothalamus departments control the mechanisms of physical thermoregulation, i.e. they are center of Heat Paths. Rear hypothalamus departments control chemical thermoregulation and are center of heat generation.
An important role in the regulation of body temperature belongs core brain. The efferent nerves of the thermoregulation center are mainly sympathetic fibers.
In the regulation of heat exchange participates and hormone mechanism, in particular, thyroid hormones and adrenal glands. Thyroid hormone - tyroxin, increasing the metabolism in the body, increases heat generation. The flow of thyroxine in the blood increases when the organism is cooled. Hormone adrenal glands - adrenalin - enhances oxidative processes, thereby increasing heat generation. In addition, under the influence of adrenaline, the vessels occurs, in particular the vessels of the skin, the heat transfer is reduced due to this.
Adaptation of the body to reduced ambient temperature. With a decrease in the ambient temperature, the reflex excitation of the hypothalamus occurs. Increased activity stimulates pituitary , the result of which is the reinforcement of thyrotropin and corticotropin, increasing the activity of the thyroid gland and adrenal glands. The hormones of these glands stimulate heat.
In this way, When cooling Protective mechanisms of the body, increasing metabolism, heat generation and reduced heat transfer.
Age features of thermoregulation. Children of the first year of life there is an imperfection of mechanisms. As a result, when the ambient temperature decreases below 15 ° C, the hypother of the children's body arises. In the first year of life there is a decrease in the heat of heat through thermal conductivity and heat emission and an increase in heat-product. However, up to 2 years, children remain thermolabile (body temperature increases after meals, at high ambient temperature). In children from 3 to 10 years, the mechanisms of thermoregulation are improved, but their instability continues to be maintained.
In pre-cheat and during puberty (puberty), when an enhanced increase in the body and the restructuring of the neurohumoral regulation of functions occur, the instability of thermoregulation mechanisms is enhanced.
In old age, there is a decrease in the formation of heat in the body compared with a mature age.
The problem of hardening the body. In all periods of life, it is necessary to order the body. Under the hardening, it is understood to increase the sustainability of the body to adverse effects of the external environment and primarily to cooling. Hardening is achieved by using natural nature factors - sun, air and water. They act on the nervous endings and vessels of human skin, increase the activity of the nervous system and contribute to the strengthening of metabolic processes. With the constant impact of natural factors, the organism is addictive. Hardening the body is effective in compliance with the following basic conditions: a) systematic and continuous use of natural factors; b) a gradual and systematic increase in the duration and strength of their impact (hardening to start using warm water, gradually reduce its temperature and increase the time of water procedures); c) hardening with the use of contrasting irritants (warm - cold water); d) an individual approach to hardening.
The use of natural harvesting factors must be combined with physical culture and sports. Well contributes to hardening the morning gymnastics in the fresh air or in the room with an open window with the mandatory exposure of a significant part of the body and subsequent water treatments (dialing, shower). Hardening is the most affordable means of improving people.
The heat flux q n through the surface S ST walls of the dryer is calculated by the heat transfer equation:
Q n \u003d k * Δt Wed * s Art,
The heat transfer coefficient K is calculated by the formula for a multilayer wall:

where δ and λ are, respectively, the thickness and coefficient of thermal conductivity of various layers of lining and thermal insulation.
Find the value of the RE:
Re \u003d V * L / υ \u003d 2.5 m / s * 1.65 m / 29 * 10 -6 m 2 / s \u003d 142241
Nu \u003d 0.66 * RE 0.5 * PR 0.33 \u003d 0.66 * 142241 0.5 * 1.17 0.33 \u003d 262.2.
The heat transfer coefficient α from the drying agent to the inner surface of the walls:
α 1 \u003d nu * λ / l \u003d 262.2 * 3.53 * 10 -2 W / (M * K) / 1.65 m \u003d 5.61 W / m 2 * k.
The total coefficient of heat transfer convection and radiation from the outer wall to the surrounding air:
α 2 \u003d 9.74 + 0.07 * (T st -t c),
where t Cp is the temperature of the outer wall, T st \u003d 40 0 \u200b\u200bs,
t B is the ambient temperature, T B \u003d 20 0 s,
α 2 \u003d 9.74 + 0.07 * (40 0 C-20 0 s) \u003d 11.14 W / m 2 * to.
In terms of gases, we select the thickness of the lining (tab. 3.1)
lining -
chamota - 125 mm
steel - 20 mm
chamot - 1.05 W / m * to
steel - 46.5 W / m * to
We find the coefficient of heat transfer:

Determine the surface of the wall s st:
S v \u003d π * d * l \u003d 3,14 * 1.6 m * 8 m \u003d 40.2 m 2,
Q n \u003d 2,581 W / (m 2 * k) * 89 0 C * 40.2 m 2 \u003d 9234 W.
The specific loss of heat into the environment is determined by the formula:
where W is the mass of moisture, removed from the dried material for 1 s.
q n \u003d 9234 W / 0.061 kg / s \u003d 151377.05 W * C / kg.
2.3. Calculator calculation when drying air
The total amount of heat C 0 is calculated by the formula:
Q 0 \u003d L * (i 1 -i 0)
Q 0 \u003d 2.46 kg / s * (159 kJ / kg +3.35 kJ / kg) \u003d 399,381kW
We calculate the average temperature pressure according to the logarithmic equation:

where Δt m \u003d t 1 -t 2n
Δt b \u003d T 1 -T 2K
t 1 - the temperature of the heating steam (equal to the saturation temperature of the steam at a given pressure).
With a pressure of 5.5 atm. T 1 \u003d 154.6 0 s (ST 550)
t 2N, T 2K - air temperature at the inlet in the calorimeter and outlet of it, T 2k \u003d 150 0 s; T 2N \u003d -7.7 0 S.
Δt b \u003d 154.6 0 s + 7.7 0 C \u003d 162.3 0 s,
Δt m \u003d 154.6 0 C-150 0 C \u003d 4.6 0 s,


The surface of the heat exchange S T calorimeter is determined by the heat transfer equation:
S T \u003d Q 0 / K ΔT Wed,
where the coefficient of heat transfer, which for finned calorifers is used depending on the mass air velocity ρ * v. Let ρ * v \u003d 3 kg / m 2 * C; Then K \u003d 30 W / m 2 * K.

We find the necessary number N to. Calrifer sections:
n k. \u003d s t / s s,
where s s is the surface of the section heat exchange.
Let's take a strand calorifer:
T. K. The actual number of sections are chosen with 15-20% reserve, then n to. \u003d 6.23 + 6.23 * 0.15 \u003d 7.2≈8 sections.
The mass speed of air in the caloriete is calculated:
where L-consumption of absolutely dry air,