Pharmaceutical Chemistry - Glushchenko N.N. The abstract of lectures on pharmaceutical chemistry is compiled for foreign students and students of the courses of course III
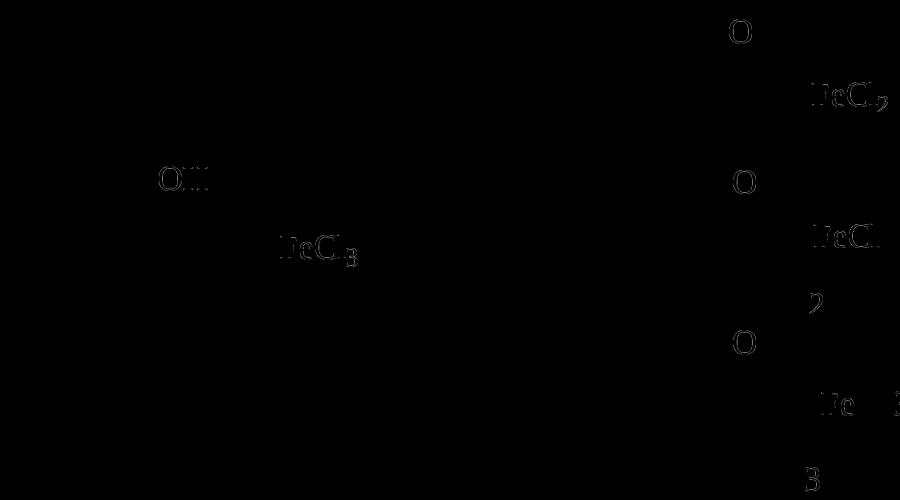
Pharmaceutical chemistry as science. History of development. Modern scientific problems
Modern scientific problems of pharmaceutical chemistry - discipline for choice, refers to the variable part of the professional GEF cycle.
The study of discipline ends with the current control in the 9th semester - an undifferentiated test.
The purpose of the development of discipline on the choice is the acquisition of in-depth knowledge of the main research problems of pharmaceutical chemistry:
creation of new drugs;
development of new and improving existing methods of drug quality control.
Pharmaceutical chemistry - Applied science, which, based on the general laws of chemical sciences, studies:
chemical nature of drugs;
methods for producing drugs;
lAN structure;
physical and chemical properties of drugs;
methods of analyzing drugs;
the relationship between the chemical structure of the LAN and the action on the body;
changes occurring during the storage of drugs;
application and form of release of drugs.
The history of the development of pharmaceutical chemistry
I. The period of Yatrochimia (XVI-XVII centuries)
Yatrochimiya, statute. Iatrochemistry (from Dr. Greek. ἰ αττός - a doctor) - the rational direction of the Alchemy XVI-XVII centuries, who wants to put chemistry to the service of medicine and the main goal of preparation of drugs.
Explained the origin of diseases by chemical processes in the human body.
The origin and development of Natrochimia, which received the greatest distribution in Germany and the Netherlands, is associated with the activities of a number of researchers.
Jan Baptist Wang Helmont (1580-1644) - Dutch naturalist, doctor. Wang Gelmont was one of the first to use silver nitrate (Lapis) for the migation of wounds, inflammation and warts. It believed that the acid of the gastric juice plays a decisive role in the digestion, and therefore offered to treat with alkalis of the disease caused by excess acids in the stomach. Introduced into the chemistry the term "gas".
Francis Silvius, he is Francois Dubua, France de la Boe
(1614-1672) - Dutch doctor, physiologist, an anata and chemist. Considered it
"Essentials" of acidic or alkaline nature and at one type of disease prescribed herring, with a different acid. He learned how to receive silver nitrate (Lapis) and use it for the migation of wounds, inflammation and warts. He opened the first chemical laboratory for analyzes at Leiden University.
(Present Name Philipp Aureol Theofrast Bombaster von Gogenheim, 1493-1541) - the famous Alchemist and a doctor of Swiss-German origin, one of the founders of yatrochimia. He believed that "not the mining of gold, and the protection of health should be chemistry."
The essence of the teachings of Paracelsa was based on the fact that the human body represents a combination of chemicals and a lack of any of them may cause a disease. Therefore, the chemical compounds of various metals (mercury, lead, copper, iron, antimony, arsenic, etc.) were used for healing Paracelles (mercury, lead. Paracels conducted a study of action on the body of many substances of mineral and vegetable origin. He enhanced a number of devices and devices for analyzing. That is why Paracelles are considered to be one of the founders of pharmaceutical analysis, and the yatrohymia - a period of the emergence of pharmaceutical chemistry.
Pharmacies in the XVI-XVII centuries. There were peculiar centers for the study of chemicals. They received and investigated substances of mineral, vegetable and animal origin. A number of new compounds were opened here, the properties and conversion of various metals were studied. This made it possible to accumulate valuable chemical knowledge, improve the chemical experiment.
II. The period of the emergence of the first chemical theories (XVII-XIX centuries)
For the development of industrial production during this period it was necessary to expand the framework of chemical research beyond the limits of yatrochimia. This led to the creation of first chemical industries and to the formation of chemical science. The second half of the XVII century. - The period of the origin of the first chemical theory is the theory of phlogiston. With it, they tried to prove that the processes of combustion and oxidation are accompanied by the release of a special substance - "Flogiston" - I. Becher (1635-1682) and Stahn (1660-1734). Despite some erroneous provisions, it was undoubtedly progressive and contributed to the development of chemical science.
In the fight against supporters of the phlogiston theory, an oxygen theory arose, which was a powerful impetus in the development of chemical thought.
M.V. Lomonosov (1711-1765) One of the first scientists in the world has proved the inconsistency of the theory of phlogiston. Despite the fact that the oxygen is not yet known, M.V. Lomonosov showed experimentally in 1756, that in the process of combustion and oxidation there is no decomposition, but accession
(1742-1786), the merit of which was also the discovery of chlorine, glycerin, a number of organic acids and other substances.
The second half of the XVIII century. There was a period of rapid development of chemistry. A large contribution to the progress of chemical science was made by the pharmacists, which were made a number of wonderful discoveries that are important both for pharmacy and chemistry.
French Pharmacist L. Voklen (1763-1829) opened new elements - chrome, beryllium.
French Chemist B. Courtois (1777-1836) discovered iodine in sea algae.
In 1807, the French pharmacist Segene allocated morphine from opium, and his compatriots Peltier and Cavent were first obtained from plant raw materials, Strychnin, Brugin and other alkaloids.
Much made for the development of pharmaceutical analysis Pharmacist Karl Friedrich Mor (1806-1879) - German chemist and pharmacist. He first applied burettes, pipettes, pharmacy scales that carry his name.
Development of pharmaceutical chemistry in Russia
The emergence of pharmacy in Russia is associated with the widespread development of traditional medicine and signs. The first cells of the pharmacy affairs in Russia were tiny shops (XIII-XV centuries), in which "languages" traded various herbs and prepared drugs from them.
By the same period (XIII-XV centuries), the occurrence of pharmaceutical analysis should be attributed, as it became necessary to test the quality of drugs. Russian pharmacies in the XVI-XVII centuries. There were peculiar laboratories for the manufacture of not only LS, but also acids (sulfur and nitric), alum, culp, sulfur cleaning, etc. Consequently, pharmacies were the landmark of pharmaceutical chemistry. The training of pharmacist personnel was carried out open in 1706 in Moscow the first medical school. One of the special disciplines in it was pharmaceutical chemistry. Many Russian chemists have been educated in this school.
The true development of chemical and pharmaceutical science in Russia is associated with the name Mikhail Vasilyevich Lomonosov (1711-1765). At the initiative of M.V. Lomonosov in 1748 was created the first scientific chemical laboratory, and in 1755 the first Russian university was opened. Together with the Academy of Sciences, these were the centers of Russian science, including chemical and pharmaceutical.
One of the numerous successors M.V. Lomonosov was a pharmacy student, and then a large Russian scientist Tovi Egorovich Lovitz (1757-1804). He first discovered the adsorption capacity of coal and
applied it for water purification, alcohol, wine-acid; Developed methods for producing absolute alcohol, acetic acid, grape sugar. Among numerous works ie The catcher is directly related to pharmaceutical chemistry has the development of a microcrystaloscopic analysis method (1798).
Decent successor M.V. Lomonosov was the largest Russian chemical scientist Vasily Mikhailovich Seryghin (1765-1826). The greatest value for pharmacy have two of its books, published in 1800: "Method to experience the purity and non-compliance of the chemical works of medicinal" and "Method of experiencing mineral waters". V.M. Severgin created the scientific basis of not only pharmaceutical, but also chemical analysis in our country.
The Encyclopedia of Pharmaceutical Knowledge is called the works of the Russian scientist Alexander Petrovich Nelyubin (1785-1858). He first formulated the scientific foundations of pharmacy, fulfilled a number of applied research in the field of pharmaceutical chemistry; Improved ways to produce chinine salts, created devices for the ether and to test arsenic. A.P. Unlubube conducted broad chemical studies of Caucasian mineral waters.
The founders of the first Russian chemical schools in Russia were
A.A. Resurrection (1809-1880) and h.h. Zinin (1812-1880).
A.A. Resurrection and H.h. Zinin played an important role in training,
in the creation of laboratories was greatly influenced by the development of chemical sciences, including pharmaceutical chemistry. A.A. Resurrection fulfilled with his students a number of studies with a direct attitude to pharmacy. They allocated alkaloid theobromin, conducted studies of the chemical structure of Kinin. Outstanding opening H.H. Zinina was a classic reaction of the conversion of aromatic nitro compounds in the amino compound.
DI. Mendeleev (1834-1907) is the creator of a periodic law and a periodic system of elements. DI. Mendeleev paid attention and pharmacy. Back in 1892, he wrote about the need "Device
in Russia of factories and laboratories for the production of pharmaceutical and hygienic preparations "in order to exemplate imports.
hexamethyleneteremin, discovered Hinoline, studying the structure of Kinin, synthesized sugar substances from formaldehyde. The world glory brought A.M. Butlerov Creation (1861) Theory of the structure of organic compounds.
Periodic system of elements D.I. Mendeleev and the theory of the structure of organic compounds A.M. Butlerov had a decisive effect on the development of chemical science and its connection with production.
At the end of the XIX century. In Russia, wide studies of natural substances were conducted. Back in 1880, long before the works of the Polish scientist
russian doctor N.I. Lunin suggested that there is in food except protein, fat, sugar "substances, indispensable for nutrition." He experimentally proved the existence of these substances, which were later called vitamins.
In 1890, E. Shatsky's book was published in Kazan. "The doctrine on plant alkaloids, glucosides and ptomaines". It addresses alkaloids, known by that time, in accordance with their classification for producing plants. The methods of extraction of alkaloids from vegetable raw materials, including the device proposed by E. Shatsky, are described.
At the turn of the XX century. In connection with the rapid development of medicine, biology and chemistry, chemotherapy arose. In their contribution to its development, both domestic and foreign scientists were made. One of the creators of chemotherapy is Russian doctor D.L. Romanovsky. It formulated in 1891 and confirmed experimentally the foundations of this science, indicating that it is necessary to look for a "substance", which, when introduced into a sick, the body will have the least harm and cause the greatest destructive effect in the pathogenic agent. This definition has kept its value to this day.
Based on developed at the end of the XIX century. German scientist P. Erlich theory, called the principle of chemical variation, many, including Russian scientists (O. Y. Magidson, M.Ya. Kraft, M.V. Rubtsov, Am Grigorovsky) created a large number of chemotherapeutic agents with antimalarial action .
The creation of sulfonamide drugs, which marked the beginning of a new era in the development of chemotherapy, is associated with the study of the azocracy tightened, open in search of drugs for the treatment of bacterial infections (Gomagk, 1930). The discovery ringed was the confirmation of the continuity of scientific research - from dyes to sulfanimamides.
For the first time, in 1928, Anglicin A. Fleming Antibiotic Penicillin was an investigator of new chemotherapeutic agents, effective in causative agents of many diseases. Works A. Fleming were preceded by the research of Russian scientists.
In 1872 V.A. Manassein established the absence of bacteria in a coastal liquid during the cultivation of green mold (Pénicillium glaucum). The antibiotic effect of mold was confirmed in 1904 by the veterinary doctor M.G. Tartakovsky in experiments with the causative agent of chicken plague. The study and production of antibiotics led to the creation of a whole branch of science and industry, made a revolution in the field of drug therapy of many diseases.
Thus, conducted by scientists in Russia at the end of the XIX century. Research in the field of chemotherapy and chemistry of natural substances laid the foundations of obtaining new effective drugs in subsequent years.
Development of pharmaceutical chemistry in the USSR
The formation and development of pharmaceutical chemistry in the USSR
it happened in the first years of Soviet power in close connection with chemical science and production. The domestic schools of chemists created in Russia are preserved, which have had a huge impact on the development of pharmaceutical chemistry.
Large schools:
chemists-organic A.E. Favorsky and N.D. Zelinsky;
researchers of the Chemistry Terpenov S.S. Network;
c.B synthetic rubber creator. Lebedev;
researchers in the region Physical and chemical methods of research N.S. Kurkinakova et al.
The center of science in the country is the Academy of Sciences of the USSR (now - Russian Academy of Sciences - RAS).
Pharmaceutical chemistry developed on the basis of fundamental theoretical studies, which were conducted in the Scientific Research Institutes of the Chemical and Medical and Biological Profile of the USSR Academy of Sciences (RAS) and AMN of the USSR (now RAMS). Academic institutions accepted directly participate in the creation of new drugs.
A.E. Chichibabin (1871-1945) - the first studies in the field of natural biologically active substances (BAV).
I.L. Knunyantz (1906-1990), O.Yu. Magidson (1890-1971) - Development of technology for the production of a domestic anti-alarium drug Akrichin.
H.A. Preobrazhensky (1896-1968) - new methods for obtaining vitamins A, E, RR were developed and implemented in the production, the synthesis of pylocarpine was implemented, and studies of the coenzymes, lipids and other Bav were studied.
V.M. Rodioni (1878-1954) - contribution to the development of studies in the field of chemistry of heterocyclic compounds and amino acids, one of the founders of the domestic industry of fine organic synthesis
and chemical-pharmaceutical Industry.
A.P. Nuts (1881-1939) - Development of methods for isolating, cleaning and determining the chemical structure of many alkaloids, which then found use as an LAN.
MM Shemyakin (1908-1970) - created the Institute of Chemistry of Natural Connections. Fundamental research in the field of chemistry of antibiotics, peptides, proteins, nucleotides, lipids, enzymes, carbohydrates, steroid hormones have been carried out. On this basis, new drugs have been created. The institute laid the theoretical foundations of the new science - bioorganic chemistry.
A.N. Nesmeyanov, A.E. Arbuzov, B.A. Arbuzov, M.I. Kabachnik, I.L. Knunyantz - research in the field of elementorganic compounds.
Development of the theoretical basis for the creation of new drugs, which are elementganic compounds.
Synthetic chemists (N.V. Chromov-Borisov, N.K. Kochetkov), microbiologists (Z.V. Ermolyeva, P. Gauz, etc.), Pharmacologists (S.V. Anichkov, V.V. , MD Mashkovsky, G.N. Pershin et al.) - created original domestic drugs.
Creation of research institutes of the pharmaceutical profile in the USSR
1920 - Research Chemical and Pharmaceutical Institute (Nichchi), in 1937 - renamed Vynchi them. S. Ordzhonikidze.
1920 - Nichchi in Kharkov.
1930 - Nichchi in Leningrad.
1932 - Nichchi in Tbilisi.
70s - Nichchi in Novokuznetsk to provide scientific and technical assistance to chemical-pharmaceutical enterprises of Siberia.
Studies of Moscow
Iodine problem was solved in our country (O. Y. Magidson, A.G. Baikov, etc.). Methods for obtaining original antimalarial preparations, sulfonamides (O.Yu. Magidson, MV Rubtsov, etc.), anti-tuberculosis funds (S.I. Sergievskaya), arsenic drugs (G.A. Kirchhof, M.Ya. Kraft and Dr.), steroid hormonal drugs (V.I. Maximov, HH Suvorov, etc.), large studies were conducted in the field of alkaloid chemistry (A.P. Orekhov). This institute is now called the Center for the Chemistry of Medicines (TCLC). The center performs research and development work and produces pharmaceutical substances.
THLS-Vernie today
Main mission:
development, preclinical research and introduction into industrial production of original medicines for the prevention and treatment of widespread diseases;
reproduction of expensive synthetic drugs used in world medical practice in order to afford it for patients in Russia;
development of original and reproduced drugs (antihistamine, hormonal, ophthalmological, anti-inflammatory, antiviral, antimicrobial, psychotropic, cardiovascular, antispasmodic, cytostatic and other drugs);
preclinical study of synthetic drugs (item
28 roszdravnadzor's letters of July 14, 2009 No. 04 and 389/09);
leading organization Scientific and technical expertise of projects of regulatory and technological documentation for the production of synthetic drugs, single and multicomponent prepared dosage forms in accordance with paragraph
4.9 and the application A to Ost 64-02-003-2002;
manufacturer of pharmaceutical substances, intermediate products and placebo (Roszdravnadzor license number FS-99-04-000667 of 06.02.2009);
more than 170 generics are reproduced, widely used in world medical practice: Akrichin, Aminazine, Dimedrol, Ibuprofen, imipramine, clofelin, lidocaine, nitrazempam, orthophene, piracetam, synaphlated, tropindol, cyclodol, cisplatin, etc.;
about 80 original domestic drugs have been developed, including those known as Azapane (Pipophhesine), Arbidol, Galanamine, Dioxide, Metacin, Metronidazole Gemisuccinate, Pyrazidol (Porlindol), Platifillain, Proksodolol, Promedol, Riodeoksol, Salazopyridazine (Mesalazin), Tetraksolin (Oxolin) , Phenkarol (chiphenadine), fivazide, emoxipin;
preclinical studies are held:
pharmacological studies, including the study of the LS operation mechanism and the study of the effectiveness of the drug in comparison with the analogues;
biological research, including primary study in vitro and in vivo compound activity;
toxicological research;
analysis of acute, chronic toxicity and pieces of preparations;
pharmacokinetic studies.
The industrial technology department of the Center for Medicinal Chemistry produces the following pharmaceutical substances:
Benzetonia chloride - antimicrobial agent;
Collargol - antiseptic agent;
Methyl ethylpyridinol hydrochloride (Emoxipin) - antioxidant;
Microsidin - antifungal agent;
Proxodol - alpha- and beta adrenoblocator;
Poparturgol (silver proteinate) - anti-inflammatory agent for local applications;
Tropindol (Tropeetron) is a controversial agent.
Vilar - All-Russian Research Institute of Medicinal and Aromatic Plants (Created in 1931)
Based on the study of plant raw materials at the institute, more than 100 drugs were developed: individual LS or the amount of substances,
medicinal fees, individual plants with different types of action:
cardiovascular;
neurotropic;
antiviral;
anti-inflammatory;
antibacterial;
wound-healing;
bronchology;
regulating the functions of the gastrointestinal tract and the urinary sector;
immunomodulving.
Bud based on vegetable raw materials (a lining and soft toning effect) has been created.
Structure of Vilar
Center of crop production;
Center of Chemistry and Pharmaceutical Technology;
Medicine Center;
Research and the educational center of biomedical technologies;
Center for Development and Research and Research and others. The main objectives of the Institute:
fundamental and priority applied scientific research in the field of life on life on molecular, cellular, fabric
and organiser levels;
development and creation of promising technologies of living systems and drugs aimed at improving the quality and life expectancy of the population;
the introduction of achievements of science and best practices in the field of agro-industrial complex, providing its innovative technological, economic and social development;
development and upgrades of own Scientific and production
GNNICLS
The State Research Institute for Standardization and Medicinal Control (GNIKIscLC) was established in 1976 to improve the quality control of drugs. The Institute implemented fundamental and applied research on the "Standardization of Medicines", including the development of standard samples (CO) and regulatory documentation (ND) on drugs, the development of quality control methods and the study of the physicochemical and biological properties of the LAN.
In 1999, GNIIscolls was reorganized in two Research Institute: Institute for the Quality of Medicinal Quality and Standardization Institute
medicines. Both of them became part of the State Scientific Center for Examination and Medicinal Control.
History of the Department of Pharmaceutical Chemistry FOO
In 1918, a decree of the Soviet government was issued about the opening of the pharmaceutical department at the Perm State University. Classes at the rate of pharmaceutical chemistry were held on the basis of the University. The founder of the Department of Pharmaceutical Chemistry is Professor Nikolai Ivanovich Krom.
1931 - the beginning of the departments of the department. In the building of the medical institute (st. K. Marx), the Department worked from 1931 to 1937.
As an independent structural unit, the department of pharmaceutical chemistry was highlighted in 1937 after a number of transformations and the allocation of the pharmaceutical department in the Perm pharmaceutical Institute. In the building on the street. Lenin, 48 Department worked from 1941 to 1965.
The main problems of pharmaceutical chemistry
I. Creating new drugs.
II. Development of new and improving existing methods of drug quality control.
By solving the problem of creating and researching new drugs in Russia:
universities;
chemical-technological institutes;
research institutes;
educational establishments;
research Institutions of the RAMS, etc.
I. Creating new medicines
Empirical search - the method of random discoveries. A variety is general screening (screening). A large amount of substances obtained are subjected to pharmacological tests on animals and identifies substances with biological activity.
Directional synthesis - provides for the preparation of LS with alleged biological activity.
Main types of directional synthesis
1. Reproduction of biogenic physiologically active substances (vitamins, hormones, enzymes, biogenic amines, etc.).
2. Detection of physiologically active metabolites and the creation of new medicines based on metabolites and antimetabolites.
Year of issue: 2004
Genre: Pharmacology
Format: Djvu.
Quality: Scanned Pages
Description:The volume of the material set forth in the textbook "Pharmaceutical Chemistry" significantly exceeds the content of the curriculum for pharmaceutical schools. The authors deliberately went to such an extension, given the examples of some foreign and domestic textbooks, where the subject is subject to the involvement of information on the latest scientific achievements. This allows the teacher to independently make the selection of the material recommended by the program in accordance with the established traditions of the educational institution. Taking into account the high level of preparation of some students, the wider presentation of the subject will help them when studying some sections.
A feature of the material presentation is the use of data of the Russian Encyclopedia of Medicines (2003), US Pharmacopoeia (USP-24), European Pharmacopoeia (EP-2002), British Pharmacopoeia (BF 2001), Scientific Editions of the Recent years and the current scientific periodicals in the chemistry of drugs (LS). The use of foreign pharmacopoeia in the preparation of the textbook is fully justified, since the domestic pharmacopoeia has not fully reissued since 1968, and the receipt of temporary pharmacopy articles by educational institutions is associated with tangible material costs. In addition, in Russia, as is well known, work is underway to implement GP (Good Practice - appropriate) methods in pharmacy at all stages of the "life" of medication. Proper pharmaceutical practice crossed the borders of the United States and Europe. Therefore, the future domestic pharmacopoeia will certainly emphasize much positive, which is achieved and is used in the countries belonging to the European Pharmacopoei community (EF) as members and observers.
It is possible that the integration of countries at all levels will facilitate the task of joining Russia to the European Pharmacopoeia, as has already done 27 states. Such unity, coordination (harmonization) of the pharmacopoeia of different countries is not by chance: the medicine that we sell or acquire has ceased to belong to one country. Substances, auxiliary substances, reagents, packaging, methods of quality control of all components, instrument for analysis - the fruit of the work of specialists from different countries. Ultimately, LS may be in the market of a completely different state. Unfortunately, currently the requirements applied in different countries to assess the safety and effectiveness of drugs differ. That is why the question of the statements of the pharmacopoeia of various states, both producing drugs, and apply them on their territory.
Non-traditional for pharmaceutical chemistry approaches were used to characterize the biological activity of medicinal substances in biological environments. Thus, the authors applied the "pH diagrams" methods and the pH-potential diagrams for acid-base and redox processes involving drugs. When describing the features of synthesis, analysis, storage conditions, therapeutic activity, fundamental laws were used, in particular, the law of active masses for equilibrium and the law of the active masses.
For the first time in the educational literature, Lal-Test included in the last pharmacopoeial publication and meets the requirements of the GMP (Good Manufacturing Practice) is described for assessing the pyrohood of injection dosage forms.
Unfortunately, some issues important for pharmaceutical chemistry remained outlining, which is explained by the restrictions on the volume of publication.
The textbook "Pharmaceutical Chemistry" is written by the team of authors representing three interrelated areas - biology, chemistry, pharmacy.
Glushchenko Natalia Nikolaevna
- Doctor of biological sciences, head. The laboratory for the impact of heavy metals on the biosystems of the Institute of Energy Problems of Chemical Physics of the Russian Academy of Sciences.
Pletcheva Tatyana Vadimovna
- Professor, Doctor of Chemical Sciences, Head of the Department of Pharmaceutical and Toxicological Chemistry of the Medical Faculty of the Russian University of Friendship of Peoples.
Popkov Vladimir Andreevich
- Professor, Doctor of Pharmaceutical Sciences, Doctor of Pedagogical Sciences, Academician Academy of Education, Head of the Department of General Chemistry of the Moscow Medical Academy. THEM. Sechenov.
The authors will be grateful for critical comments and wishes to improve the content of the textbook.
The textbook "Pharmaceutical Chemistry" is intended for students of secondary medical schools and colleges, students in the specialty 0405 "Pharmacy". Separate sections of the textbook can be used by students of universities and listeners of faculties of advanced training.
"Pharmaceutical Chemistry"
Introduction to the chemistry of medicines
The content of pharmaceutical chemistry
- Communication of pharmaceutical chemistry with other sciences
- The main terms and concepts used in pharmaceutical chemistry
- Classification of medicines
- Drug sources
- The main directions of searching and creating medicinal substances
- Criteria for the quality of medicines
- Standardization of medicines. Control and permitting system for ensuring the quality of medicines
- Medicinal analysis methods
- General information about methods and tests of drugs for toxicity, sterility and microbiological purity
- Determination of bioequivalence and bioavailability of drugs by kinetic methods
- The expiration date and stabilization of medicines
- INTERAPTER CONTROL OF DICES
Drugs s-elements
- General group characteristics
- Chemistry of magnesium medicines
- Calcium medicines chemistry
- Chemistry of medicines barium
- Medicinal products of R-elements of the group VII
- Drugs P-elements VI Groups
- Drugs V Groups
- Drugs P-elements IV Group
- Drugs P-elements III Groups
- Drugs of D-Elements I Group
- Drugs of D-Elements of Group II
- D-elements of the group VIII group
- Drug F-Elements
Homeopathic medicines
Organic Medicinal Chemistry
Drugs of organic nature and features of their analysis
- Classification
- Analysis
- Alcohol
- Aldehydes
- Carbohydrates
- Esters
- Carboxylic acids. Aminocarboxylic acids and their derivatives
Carbocyclic drugs
- Aminospirts of aromatic row
- Phenols, Hinons and their derivatives
- Aromatic acids, hydroxy acids and their derivatives
- Aromatic amino acids
- Aromatic acetamin production
- Furan derivatives
- Derivatives Pyrazola.
- Derivatives of imidazole.
- Pyridine derivatives
- Pyrimidine derivatives
- Derivatives Tropane
- Derivatives of Chinolina
- Derivatives of isoquinoline
- Pyrin derivatives
- Derivatives of Isoalloxazin
- Antibiotics with Azetidinov Sound (R Lactamida)
- Antibiotics tetracycline row
- Antibiotics - aminoglycosides
- Antibiotics of aromatic series - derivatives of nitrophenylalkalamnins (group of Levomycetin)
- Antibiotics macrolid and azalida
Bibliography
Information on specialty
The Department of Organic Chemistry of the Chemical Technology Faculty prepares graduate specialists in the specialty 04.05.01 "Fundamental and Applied Chemistry", specializations "Organic Chemistry" and "Pharmaceutical Chemistry". The team of the department is highly qualified teachers and researchers: 5 doctors of sciences and 12 candidates of Chemical Sciences.
Professional activities of graduates
Graduates are preparing for the following types of professional activities: research, scientific and manufacturing, pedagogical, design and organizational and managerial. The specialist-chemical specialty "Fundamental and Applied Chemistry" will be ready to solve the following professional tasks: planning and working, which includes the study of the composition, structure and properties of substances and chemical processes, the creation and development of new promising materials and chemical technologies, solving fundamental and applied tasks in the field of chemistry and chemical technology; preparation of the report and scientific publications; Scientific and pedagogical activities in the university, in an average special educational institution, in high school. Students who have been involved in scientific work can pass internship, take part in scientific conferences, competitions and competitions of various levels, as well as to submit the results of scientific work for publication in Russian and foreign scientific journals. At the disposal of students there are chemical laboratories equipped with modern equipment and computer class, with the necessary literature and access to full-text electronic databases.
Experts will be:
- own chemical experiment skills, the main synthetic and analytical methods of obtaining and researching chemicals and reactions;
- submit the main chemical, physical and technical aspects of chemical industrial production, taking into account commodity and energy costs;
- own work skills on modern educational and scientific equipment when conducting chemical experiments;
- have experience in serial equipment used in analytical and physicochemical studies (gas-liquid chromatography, infrared and ultraviolet spectroscopy);
- to own the methods of registration and processing the results of chemical experiments.
- To own the skills of planning, setting and conducting chemical experiments in the field of fine organic synthesis to obtain substances with specified useful properties
Students acquire knowledge in the basics of inorganic chemistry, organic chemistry, physical and colloidal chemistry, analytical chemistry, planning of organic synthesis, chemistry of alicyclic and frame compounds, catalysis in organic synthesis, chemistry of elementantorganic compounds, pharmaceutical chemistry, modern methods of analyzing and controlling the quality of drugs , Fundamentals of medical chemistry, Fundamentals of pharmaceutical preparations, the basics of pharmaceutical analysis. In the course of practical studies, students receive work skills in a modern chemical laboratory, master the methods for obtaining and analyzing new compounds. Students own work skills on a gas-liquid chromatograph, an infrared spectrophotometer, an ultraviolet spectrophotometer. Students pass in-depth study of a foreign language (for 3 years).
In the process of learning, students master the methods of work on analytical equipment of the Department "Organic Chemistry":
Chromato masses Spectrometer Finnigan Trace DSQ
NMR Spectrometer JEOL JNM ECX-400 (400 MHz)
HPLC / MS with a high-resolution time mass spectrometer with an ESI and DART ionization source, with diodeal and fluorimetric detectors
Preparative Flash Chromatography System with UV and ELSD Reveleris X2 Detector
Infrared-Fourier Spectrometer SHIMADZU IRAFFINITY-1
Waters Liquid Chromatograph with UV and Refractometric Detectors
Differential Scanning Calorimeter TU INSTRUMENTS DSC-Q20
Automatic C, H, N, S Analyzer EUROVECTOR EA-3000
Scanning Spectrofluorimeter Varian Cary Eclipse
Auto Polarimeter Autopol V Plus
Automatic device for determining the melting point OPTIMELT
High Performance Computing Station
In the process of learning, introductory and chemical-technological practices in the laboratories of enterprises are envisaged:
- CJSC "All-Russian Research Institute of Organic Synthesis of NK";
- OJSC "Meadunevian Research Institute for Oil Refining" NK Rosneft;
- CJSC "Tarkett";
- Samara CHP;
- OJSC "Syzransky refinery" of the NK Rosneft;
- OJSC Hyprovostokneft;
- OJSC "Plant Aviation Bearings";
- LLC "Novokuibyshevsky plant oils and additives" of the NK Rosneft;
- CJSC "Neftekhimia"
- LLC "Pranafarm"
- OOO "OZON"
- OJSC "Electrical"
- FSUE SNPRCTS
- "TsSKB progress"
- OJSC "Baltika"
- PJSC "SIBUR Holding", Tolyatti
Students who have been involved in scientific work may pass internships, take part in scientific conferences, competitions and competitions of various levels, as well as to submit the results of scientific work for publication in Russian and foreign scientific journals. Specialists who were trained in the specialty "Fundamental and Applied Chemistry" are in demand in laboratories of state scientific centers and private companies, in research and analytical laboratories of various industries (chemical, food, metallurgical, pharmaceutical, petrochemical and gas producing), in expert and criminalistic laboratories; in customs laboratories; diagnostic centers; Sanitary and epidemiological stations; environmental control organizations; certification test centers; enterprises of the chemical industry, black and non-ferrous metallurgy; in educational institutions of the system of secondary vocational education; labor protection and industrial sanitation; Meteorological stations.
Assigns qualifications "Chemist. Chemistry teacher "on the specialization" Organic Chemistry "or" Pharmaceutical Chemistry ". Enrollment according to the results of EGE: Chemistry, Mathematics and Russian. Little time: 5 years (in person). Perhaps admission to graduate school.
General pharmaceutical chemistry.
The subject and tasks of pharmaceutical chemistry.
Pharmaceutical Chemistry (FC) - Science, which studies methods for obtaining,
buildings, physical and chemical properties of medicinal substances; the relationship between their chemical structure and the effect on the body; Methods for controlling the quality of drugs and changes occurring during their storage. Tasks facing it are solved with the help of physical, chemical and physicochemical methods of research used both for synthesis and for analyzing medicinal substances. FC is based on theory and laws of related chemical sciences: inorganic, organic, analytical, physical and biological chemistry. It is closely related to pharmacology, biomedical and clinical disciplines.
Terminology in FC
The object of study of FC is pharmacological and drugs. The first of these are a substance or a mixture of substances with the established pharmacological activity that are subject to clinical trials. After clinical trials and obtaining positive results of funds are approved by the pharmacological and pharmacopoeial committees for use and is obtained by the name of the drug. A medicinal substance is a substance that is an individual chemical compound or a biological substance. The dosage form is a convenient condition attached to the drug at which the necessary therapeutic effect is achieved. It includes powders, tablets, solutions, ointment candles. A dosage form made by a certain enterprise and received a corporate name is called the drug.
Sources of drugs
Medicinal substances are in nature are divided into inorganic and organic. They can be obtained from natural sources and synthetically. Raw materials for inorganic substances can be rock formations, gases, water seas, waste production, etc. Organic medicinal substances are obtained from oil, coal, combustible shale, gases, plant tissues, animals, microorganisms, etc. sources. In recent decades, the amount of drugs obtained synthetically increased dramatically.
Often the full chemical synthesis of many compounds (alkaloids, antibiotics, glycosides, etc.) technically complex and new methods of obtaining drugs are technically complex: semi-seintez, biosynthesis, genetic engineering, tissue culture and more. With the help of semi-synthesis, drugs from semi-products of natural origin are obtained, for example Semi-synthetic penicillins, cephalosporins, etc. Biosynthesis is the natural synthesis of the final product with alive organisms based on natural intermediates.
The essence of genetic engineering is to change genetic programs of microorganisms by introducing genes into their DNA encoding biosynthesis of certain drugs, such as insulin. Culture of tissues is a reproduction in artificial conditions of animals or plants, which become raw materials for the production of drugs. Hydrobionts, vegetable and animal organisms of the seas and oceans are also used to develop the latter.
Classification of medicinal substances.
There are two types of classification of a large amount of medicinal substances: pharmacological and chemical. The first of them share medicinal substances into groups, depending on the mechanism of action on individual organs and organism systems (central nervous, cardiovascular, digestive, etc.). Such a classification is convenient for use in medical practice. Its disadvantage is that in one group there may be substances with a different chemical structure, which makes it difficult to unify the methods of their analysis.
According to the chemical classification, drugs are divided into groups, based on the generality of their chemical structure and chemical properties, regardless of pharmacological action. For example, pyridine derivatives have different effects on the body: nicotinamide is vitamin RR, dietylamide nicotine acids (Cordiamine) stimulates the central nervous system, etc. Chemical classification is convenient because it allows us to identify the dependence between the structure and mechanism of the action of medicinal substances, and also allows you to unify the methods of their analysis. In some cases, a mixed classification is used, which makes it possible to use the benefits of the pharmacological and chemical classification of drugs.
Requirements for drugs.
The quality of the drug is determined in appearance, solubility, the establishment of its authenticity, the degree of purity and the quantitative determination of the content in the preparation of pure substance. The complex of these indicators is the essence of pharmaceutical analysis, the results of which must comply with the requirements of the State Pharmacopoeia (GF).
The authenticity of the medicinal substance (confirmation of the identity of it) is established with the help of chemical, physical and physico-chemical research methods. Chemical methods include reactions to the functional groups included in the drug structure, characteristic of this substance: they, according to GF, are the reactions to the amines aromatic primary, ammonium, acetates, benzoates, bromide, bismuth, iron and oxide, iodides, potassium, calcium, Carbonates (bicarbonates), magnesium, arsenic, sodium, nitrates, nitrites, mercury oxide, salicylates, sulfates, sulphites, tartrates, phosphates, chlorides, zinc and citrates.
Physical methods for determining the authenticity of the drug include its definition: 1) physical properties: aggregate state, color, odor, taste, shapes of crystals or form of amorphous substance, hygroscopicity or degree of weathering in air, volatility, mobility and flammability and 2) physical constants: temperatures Melting (decomposition) and solidification, density, viscosity, solubility in water and other solvents, transparency and degrees of turbidity, painting, ash, not soluble in hydrochloric acid and sulphate and volatile substances and water.
Physico-chemical methods of authentication are to apply instruments for chemical analysis: spectrophotometers, fluorometers, fiery photometers, chromatography equipment, etc.
Impurities in medicines and their sources.
Many drugs contain certain impurities of foreign substances. Exceeding them can cause an undesirable effect. The reasons for entering impurities to drug substances may be insufficient cleaning of the feedstock, side products of synthesis, mechanical contamination, impurities of materials from which the equipment is made, violating the storage conditions.
The GF requires either the complete absence of impurities, or admits the maximum allowable limit of them defined for this drug, which does not affect the quality and therapeutic medication effect. To determine the permissible limit of impurities of the GF, reference solutions are provided. The result of the reaction to one or another admixture is compared with the result of the reaction carried out with the same reagents and in the same volume with a reference, standard solution containing a permissible amount of impurity. Determining the degree of purity of the drug includes a test for: chlorides, sulfates, ammonium salts, calcium, iron, zinc, heavy metals and arsenic.
region. State Pharmacopoeia of the USSR (USSR GF)
GF of the USSR is a collection of mandatory nationwide standards and regulations normalizing the quality of medicinal substances. It is based on the principles of Soviet health and reflects modern achievements in the field of pharmacy, medicine, chemistry and other related sciences. The Soviet pharmacopoeia is a national document, it reflects the social essence of the Soviet health, the level of science and culture of the population of our country. The State Pharmacopoeia of the USSR has a legislative character. Its requirements for drugs are mandatory for all enterprises and institutions of the Soviet Union, which are manufactured, stored, control the quality and use drugs.
The first edition of the Soviet pharmacopoeia, called the VII edition of the USSR State Pharmacopoeia (GF VII), was put into effect in July 1926. To create it in 1923, a special pharmacopoeial commission chaired by the RSFSR was formed in the People's Commissariat of Health. A. E. Chichibabin. The first Soviet pharmacopoeia was different from previous editions with an increased scientific level, the desire for a possible replacement of medicines manufactured from imported raw materials into drugs of domestic production. Higher requirements were presented in GF VII not only to drugs, but also to products used for their manufacture.
Based on these principles in the GF VII, 116 articles on new drugs were included and 112 articles were excluded. Significant changes were made to the requirements for the quality control of drugs. A number of new methods of chemical and biological standardization of drugs were provided, 30 common articles are included in the form of applications, describes some common reactions used to determine the quality of drugs, etc. Organoleptic control of many drugs was first replaced by more objective physicochemical methods, biological control methods were introduced.
Thus, in GP VII, paramount attention was paid to improving the quality control of drugs. This principle found its further development in subsequent publications of the pharmacopy.
In 1949, the VIII edition was published, and in October 1961 - IX edition of the USSR State Pharmacopoeia. By this time, new groups of highly efficient drugs were created (sulfonamides, antibiotics, psychotropic, hormonal and other drugs), which demanded the development of new pharmaceutical analysis methods.
X The publication of the State Pharmacopoeia (GF X) was enacted from July 1, 1969. It reflected new successes of domestic pharmaceutical and medical science and industry.
The principal difference of GP IX and GF X is the transition to a new international terminology of drugs, as well as a significant update of both the nomenclature and the methods of controlling the quality of drugs.
In GF X, the requirements for the quality of drugs are significantly increased, the methods of pharmacopoeia analysis are improved, the scope of the application of physicochemical methods has been expanded. Numerous general articles, reference tables and other materials included in GF X reflected the requirements necessary to assess the qualitative and quantitative characteristics of medicines.
The State Pharmacopoeia of the USSR X edition includes 4 parts: "Introductory part"; "Preparations" (private and group articles); "General methods of physico-chemical, chemical and biological research"; "Applications".
In the "introductory part" the general principles of constructing and the procedure for using the GF X are set out, compilers are indicated, changes that distinguish the GF X from GP IX, List A and the list of medicinal substances.
The XF X contains 707 articles on drugs (in GP IX was 754) and 31 group items (in GP IX was 27). The update of the nomenclature by 30% was due to the exclusion of drugs discontinued, as well as having limited use. The quality of the latter is set in accordance with the requirements of GP IX.
Compared, GP IX increased from 273 to 303 by the number of individual (synthetic and natural) drugs, from 10 to 22 antibiotics preparations, for the first time in GF, radioactive drugs are included. Among the drugs included in the GF, new cardiovascular, psychotropic, gangli-blocking, antimalarial, anti-tuberculosis agents, drugs for the treatment of malignant neoplasms, fungal diseases, new drugs for anesthesia, hormonal preparations, vitamins. Most of them were first obtained in our country.
"Preparations" - the main part of the XF X (p. 39-740). In 707 articles set forth the requirements for the quality of medicines (quality norms). Each drug in accordance with the requirements of the pharmacopoeia is subject to testing physical properties, authentication, purity test and determining the quantitative content of the drug. In GF X, the structure of articles reflecting the control sequence is detailed. The "Properties" section is replaced by two sections: "Description" and "solubility". The description of authentication reactions for 25 ions and functional groups is reduced to one general article, and in private articles there are references.
Changed the order of the articles. For the first time in the GF x of the article on finished dosage forms are located after articles on the appropriate drug. In most articles of GF X there is a rubric indicating the pharmacological effect of the drug. Details of higher doses of drugs are deployed at various methods of administration.
In the third part of the GF X "General Methods of Physico-Chemical, Chemical and Biological Research", a brief description of the methods used for pharmacopoeia analysis are presented with information on reagents, titted solutions and indicators.
Applications to GF X contain reference tables of atomic masses, densities, constants (solvents, acids, bases) and other qualitative indicators of drugs. This also includes tables of higher one-time and daily doses of poisonous and potent drugs for adults, children, as well as animals.
After entering the light of the edition of the State Pharmacopoeia, the USSR Ministry of Health is allowed to use a number of new highly efficient drugs in medical practice. Many of them are first developed by scientists from our country. At the same time, ineffective drugs are excluded, a change in which more modern funds came. Therefore, there is a need to create a new XI edition of the State Pharmacopoeia of the USSR, which is preparing at present. Research institutions and enterprises of the Ministry of Health of the USSR, the Ministry of the Medical Industry and other departments are involved in this work. The new state pharmacopoeia will reflect the modern achievements in the field of pharmaceutical analysis and improving the quality of medicines.
National and Regional Pharmacopoeia
Systematically after 5-8 years, there are issues of national pharmacopy such major capitalist states, as the United States, United Kingdom, France, Germany, Japan, Italy, Switzerland and some others. Published in 1924-1946. Pharmacopoeia Greece, Chile, Paraguay, Portugal, Venezuela have already lost their meaning.
Along with Pharmacopuses, in some countries, collections of officials of the US National Forms, the British Pharmaceutical Code are periodically published. They are normalized by the quality of new drugs that have not included in the pharmacopoeia or in earlier editions of Pharmacopy.
The first experience of creating a regional pharmacopoeia was carried out by Scandinavian countries (Norway, Finland, Denmark and Sweden). The published Scandinavian pharmacopoeia since 1965 acquired a legislative character for these countries.
Eight Western European States (United Kingdom, Germany, France, Italy, Belgium, Luxembourg, the Netherlands and Switzerland), which are members of the EEC (European Economic Community), created a pharmacopoeia commission in 1964. She prepared and in 1969 issued the first first, and in 1971 the second volume of pharmacopoeia UES (in 1973, an addition to these publications was released). In 1976, the Pharmacopa EEC was recognized as Scandinavian countries, Iceland and Ireland. Pharmacopoeia UES has a legislative character, but does not replace the national pharmacopoeia of these countries.
Regional pharmacopoeia contribute to the unification of the nomenclature and requirements for the quality of medicines obtained in various countries
Quality control of drugs in pharmacies
Inportal quality control of drugs includes not only analytical control, but also a system of activities that provide proper storage, preparation and vacation drugs. It is based on strict compliance with the pharmaceutical and sanitary regime in the pharmacy. It is especially closely necessary to carry out the rules for storing medicines, the technology of preparing injection solutions, concentrates and eye drops.
For internal quality control of drug quality in pharmacies, analytical cabinets or analytical tables equipped with the necessary devices, reagents, reference and special literature should be. Inportal control is carried out by analysts, which are included in the state of large pharmacies, as well as technologists-technologists, whose duties include testing the quality of drugs. They have an equipped workplace on the assistant table or next to it. The head of the pharmacy and his deputies lead the work on the quality control of drugs. They must be owned by all types of intensive control, and in small pharmacies themselves to perform the functions of a diaproof analytics or pharmacist technologist.
Direct analytical control in the pharmacy includes three main directions: quality control of medicinal substances coming from industry, quality control of distilled water and various types of quality control of dosage forms manufactured in a pharmacy.
Drugs entering the pharmacy from the industry, regardless of the presence of the SIL stamp controlled by identity. Preparations, quickly changing during storage, at least once a quarter are sent to check in control and analytical laboratories.
Systematic control over the benignness of distilled water in the pharmacy ensures the quality of the preparation of all liquid dosage forms. Therefore, distilled water is controlled in each cylinder on the absence of chlorides, sulfates T solo calcium. Even higher requirements are presented to water used to prepare injectable solutions. Its on the absence of regenerating substances, ammonia, carbon dioxide. At least once a quarter of the pharmacy directs distilled water for complete analysis to a control and analytical laboratory, and twice a year to a sanitary and bacteriological laboratory to verify the absence of pollution by microflora.
Internal control of dosage forms manufactured in pharmacies are exposed. There are several types of controls: written, organoleptic, polling, physical and chemical. A written, organoleptic, questionnaire and physical control carries out, as a rule, a provisional technologist after producing a pharmacist at least 5 drugs, and chemical control is a provisional analyst.
All drugs manufactured in any pharmacy are exposed to written control. The essence of written control lies in the fact that the pharmacist after the preparation of the medicine writes the name and the total mass of each ingredient or the content of each concentrate in a special form. Then the form together with the recipe is transmitted to test the provider-technologist. Filled blanks are stored in a pharmacy for 12 days.
Organoleptic control includes checking the appearance (color, mixingness of mixing), odor and taste of drugs, lack of mechanical pollution. All medicines prepared for internal use for internal use, and selectively prepared for adults (eliminating drugs containing ingredients belonging to the list a) are checked.
Interview control performs a provisional technologist. He calls the ingredient, and in complex drugs the content of the first ingredient. After that, the pharmacist calls all other ingredients and their quantities. If concentrates were used for making medication, then the pharmacist lists them with an indication of percentage. Interview control is carried out immediately after the manufacture of drugs, if they are intended for injections or in their composition include the drugs of the list A. When doubt, the questionnaire control is an additional type of control as a manufactured drug.
Physical control is to test the total volume (mass) of the prepared medication or the mass of its separate doses. Controlled 5-10% of the number of doses prescribed in the recipe, but at least three doses. Physical control is carried out selectively, periodically throughout the day. Together with physical control, it is verified the correctness of the verification of the designation of drugs and compliance with the packaging by the physicochemical properties of the ingredients included in the dosage form.
Chemical control includes high-quality and quantitative chemical analysis of drugs prepared in a pharmacy. All injectable solutions are subjected to high-quality chemical analysis (prior to their sterilization); eye drops; each series of concentrates, semi-finished products and internal preparation; Medicinal preparations coming from stocks in assistant; children's dosage forms; Medications containing the drugs of the list A. Selectively control the medicines made according to individual impurities.
To perform high-quality analysis, the drip method is used mainly using the tables of the most characteristic reactions.
oh practical work it is necessary to study the foundations of general pharmaceutical chemistry and methods for the study of high-quality and quantitative research of substances most common in veterinary practice.
The list of medicines to be quantitative analysis depends on the presence of a provisional analytics in the pharmacy. If it is in the state of the pharmacy, then all drugs for injection (before sterilization) are quantitative analysis; Eye drops (silver-containing nitrate, atropine sulfate, dikaine, ethylmorphine Pilocarpine hydrochloride); Sulfate atropine solutions for internal use; All concentrates, semi-finished products and intrapaid billets. The rest of the medicines are analyzed selectively, but every day every pharmacist. First of all, they control the drugs used in children's and eye practice, as well as containing the drugs of the list A. Perishable drugs (hydrogen peroxide solutions, ammonia and formaldehyde, lime water, ammonia-anise drops) are analyzed at least once a quarter.
If there is no analyst analyst, but there are two or more pharmacists in the pharmacy, then solutions for injection (prior to sterilization), containing novocaine, atropine sulfate, calcium chloride, sodium chloride, glucose, are quantitative analysis; Eye drops containing silver nitrate, atropine sulfate, pylocarpine hydrochloride; all concentrates; solutions of hydrochloric acid. Perishable drugs from these pharmacies are sent to check in control and analytical laboratories.
Qualitative and quantitative analysis in pharmacies of category with one pharmacist in the state and in pharmacy points of the first group are subject to injections for injections containing novocaine and sodium chloride; Eye drops containing atropine sulfate and silver nitrate.
The procedure for assessing the quality of drugs manufactured in pharmacies, and the norms of permissible deviations in the manufacture of drugs are established by order on the Ministry of Health of the USSR No. 382 of September 2, 1961. To assess the quality of the medicines made, the terms are used: "satisfies" or "does not satisfy" the requirements of the USSR GF, FS , WFS or instructions of the USSR Ministry of Health.
Features of pharmaceutical analysis.
Pharmaceutical analysis is one of the main sections of pharmaceutical chemistry. It has its own specific features that distinguish it from other types of analysis. Onizance in the fact that the study subjected substances of various chemical nature: inorganic, element-humanganic, radioactive, organic compounds from simple aliphatic to complex natural biologically active substances. Extremely wide range of concentrations of analyzed substances. The objects of pharmaceutical research are not only individual medicinal substances, but also mixtures containing different number of components. The amount of drugs used every year increases. This leads to the need for the development of new ways to analyze and unification already known.
Continuously increasing the quality of medicines dictates the need to continuously improve pharmaceutical analysis. The requirements of both the benignness of medicinal substances and quantitative content are growing. This causes the need for widespread use of not only chemical, but also more sensitive physicochemical methods to assess the quality of drugs.
Pharmaceutical analysis impose high demands. It should be sufficiently specific and sensitive, accurate in relation to the regulations caused by the USSR GF, WFS, FS and other NTD, to be carried out in short periods of time using the minimum amounts of the test drugs and reagents.
Pharmaceutical analysis, depending on the tasks delivered, includes various forms of quality control of drugs: pharmacopoeia analysis, postal control of drug production, analysis of individual manufacturers, express analysis in pharmacy and biopharmaceutical analysis.
An integral part of the pharmaceutical analysis is a pharmacopoeia analysis. It represents a set of ways to study drugs and dosage forms set forth in the State Pharmacopoeia or other regulatory and technical documentation (WFS, FS). Based on the results obtained in the implementation of pharmacopoeia analysis, a conclusion is made on the compliance of the drug with the requirements of the USSR GF or other regulatory and technical documentation. When deviating from these requirements, the medicine is not allowed.
The implementation of pharmacopoeia analysis allows you to establish the authenticity of the drug, its benignness, to determine the quantitative content of the pharmacologically active substance or ingredients included in the dosage form. Despite the fact that each of these stages has its own specific goal, they cannot be considered isolated. They are interrelated and mutually complement each other. For example, the melting point, solubility, pH of an aqueous solution, etc. These criteria are both authenticity and benignness of the medicinal substance.
The GF X describes the methods of appropriate tests in relation to a particular pharmacopy drug. Many of these techniques are identical. To summarize a large amount of private information on pharmacopoeia analysis, the main criteria for pharmaceutical analysis and general principles of authenticity tests, benignness and quantitative determination of medicinal substances will be considered. In some sections, the state and prospects for the use of physicochemical and biological methods in the analysis of drugs are considered.
Department of Pharmacy
Organic drugs.
Aromatic compounds.
Brief summary of lectures.
Nizhny Novgorod
UDC 615.014.479
Organic drugs. Aromatic compounds. A brief summary of lectures - Nizhny Novgorod: Publishing House of the Nizhny Novgorod State Medical Academy, 2004.
A brief summary of the lectures for pharmaceutical chemistry is compiled for foreign students and students of the Course III training form.
The properties of aromatic organic substances used as drugs are presented, the methods of obtaining, authentication and quantifying these substances are presented.
Compiled in accordance with the exemplary program under pharmaceutical chemistry and order of the Ministry of Health of the Russian Federation No. 93 of 31.03.97 "On the phased introduction since 1997 the final state certification of graduates of higher medical and pharmaceutical universities."
Recommended for publication by the Council of the Nizhny Novgorod State Medical Academy.
Compilers: Melnikova N.B., Kononova S.V., Pegova I.A., Popova T.N., Ryzhova E.S., Kulikov M.V.
.
Reviewers: Professor of the Department of "Biotechnology, Physical and Analytical Chemistry" of the Nizhny Novgorod State Technical University, D.H.N. Arbatsky A.P.; Chief Technologist OJSC NIPARM, Ph.D. Jen F.h.
© NB Melnikova,
S.V. Kononova,
I.A. Pegova,
So-called Popova,
E.S. Ryzhova,
M.V. Kulikov, 2004.
| Aromatic compounds (arena), general characteristics. | 4 |
|
| Phenols, quinones and their derivatives. | 6 |
|
| Naftoquinone derivatives (vitamins of group K). | 24 |
|
| Para-aminophenol (paracetamol) derivatives. | 31 |
|
| Aromatic acids and their derivatives. Salicylic acid esters. Amite salicylic acid. | ||
| Couple, ortho-aminobenzoic acids and their derivatives. | 51 |
|
| Arylalkylamines, hydroxyphenylaclamines and their derivatives. | 70 |
|
| Benzillsulfanimamides and their derivatives. | 92 |
|
| Literature | 103 |
Aromatic compounds (arena).
General characteristics.
Arena - Compounds with a planar cyclic aromatic system in which all cycle atoms are involved in the formation of a single conjugate system, comprising according to the Hyukkel rule (4N + 2) π-electrons.
The classification of arena is carried out according to functional groups, because They allow to analyze drugs and determine the physiological effect.
Communication of the structure with physiological activity.
resorcin - purple-black, turning into purple;
hexesterrol (synestrol) - red-purple, turning into cherry.
Complexation reaction with iron ions.
4.1.



Complexes painted:
phenol - blue color;
resorcin - blue-purple color;
salicylic acid - blue-purple or red-purple color;
osalmid (Oxafenamide) - red-purple color;
sodium para-aminoaliculate - red-purple color;
hinosol - bluish green.
The reaction is a pharmacopoeia for most phenolic compounds.
Electrophilic replacement reactions - s E hydrogen atom in aromatic ring (bromination, condensation with aldehydes, combination with diazonia salts, nitration, nitrosing, iodization, etc.). The ability of phenols to enhance the electrophile replacement reaction is due to the interaction of the vulnerable electron pair of an oxygen atom with π-electrons of the benzene ring. Electronic density shifts towards the aromatic ring. The greatest excess of electron density is observed in carbon atoms in about- and n.- positions with respect to phenolic hydroxyl (orient orientation).
5.1. Halogenation reaction (bromination and iodization).

With an excess of bromine, oxidation occurs:

The reaction of phenol bromination depends on the nature and position of the substituents.
Similarly, iodization occurs, for example:

5.1.2. In the presence of deputies about- and n.- the positions of the aromatic ring to the reaction are joined by unsubstituted hydrogen atoms of the aromatic ring.

5.1.3. If in about- and n.-
the positions with respect to phenolic hydroxyl are a carboxyl group, then decarboxylation occurs under the action of excess bromine: 
5.1.4. If the compound contains two phenolic hydroxyl into m-position, then when the bromine action is formed tribrome derivatives (consistent orientation): 
5.1.5. If two hydroxyl groups are located relative to each other in about- or n.-
provisions, the reaction of bromination does not proceed (inconsistent orientation) 
5.2. Reactions condensation
5.2.1. With aldehydes.

5.2.2. The reaction of phenols with chloroform (CHCl 3) with the formation of aurin dyes.

Aurines are painted:
phenol - yellow color;
timol - yellow, turning into purple;
resorcin - red-purple color.
5.2.3. With acid anhydrides.
A. Fluoresian formation reaction (condensation of resorcin with phthalic anhydride). 
yellow-red solution with green fluorescence (pharmacopoeial reaction to resorcin)
B. The reaction of the formation of phenolphthalein (condensation of phenol with phthalic anhydride).

With a large excess of the alkali, a three-seated sodium salt is formed.
The condensation of thymol with phthalic anhydride goes similarly to the reaction of the formation of phenolphthalein, thymolftaleine is formed, having a blue staining in an alkaline medium.
5.3. Nuting reaction

5.4. Azosochetation reaction of phenols with salt diazonia in an alkaline environment.

In case of phenol
