Heat of combustion of associated gas. Natural gas and its calorific value under domestic conditions
Read also
The amount of heat released during the complete combustion of a unit of the amount of fuel is called the calorific value (Q) or, as they sometimes say, the calorific value, or calorific value, which is one of the main characteristics of the fuel.
The calorific value of gases is usually referred to 1 m 3, taken under normal conditions.
In technical calculations, normal conditions mean the state of the gas at a temperature equal to 0 ° C, and, at a pressure of 760 mmHg Art. The gas volume under these conditions is denoted nm 3(normal cubic meter).
For industrial gas measurements in accordance with GOST 2923-45, the normal conditions are taken as a temperature of 20 ° C and a Pressure of 760 mmHg Art. The volume of gas attributed to these conditions, in contrast to nm 3 will call m 3 (cubic meter).
Calorific value of gases (Q)) expressed in kcal / nm e or in kcal / m 3.
For liquefied gases, the calorific value is referred to 1 kg.
Distinguish between higher (Q in) and lower (Q n) calorific value. The gross calorific value takes into account the heat of condensation of water vapor generated during fuel combustion. The net calorific value does not take into account the heat contained in the water vapor of the combustion products, since water chests do not condense, but are carried away with the combustion products.
The concepts of Q in and Q n refer only to those gases, the combustion of which emits water vapor (these concepts do not apply to carbon monoxide, which does not produce water vapor during combustion).
During condensation of water vapor, heat is released, equal to 539 kcal / kg. In addition, when the condensate is cooled to 0 ° C (. Or 20 ° C), respectively, heat is released in the amount of 100 or 80 kcal / kg.
In total, more than 600 heat is released due to condensation of water vapor. kcal / kg, which is the difference between the gross and net calorific value of the gas. For most gases used in urban gas supply, this difference is 8-10%.
The calorific values of some gases are given in table. 3.
For urban gas supply, gases are currently used, which, as a rule, have a calorific value of at least 3500 kcal / nm 3. This is explained by the fact that in urban conditions gas is supplied through pipes over considerable distances. If the calorific value is low, a large amount must be fed. This inevitably leads to an increase in the diameters of gas pipelines and, as a consequence, to an increase in metal investments and funds for the construction of gas networks, and in the following: and to an increase in operating costs. A significant disadvantage of low-calorific gases is that in most cases they contain a significant amount of carbon monoxide, which increases the danger when using gas, as well as when servicing networks and installations.
Gas with a heating value of less than 3500 kcal / nm 3 most often used in industry, where it is not required to transport it over long distances and it is easier to organize incineration. For urban gas supply, it is desirable to have a constant calorific value. Fluctuations, as we have already established, are allowed no more than 10%. A large change in the calorific value of a gas requires a new adjustment, and sometimes a change in a large number of standardized burners for household appliances, which is associated with significant difficulties.
Combustible gas classification
For gas supply to cities and industrial enterprises, various combustible gases are used, differing in origin, chemical composition and physical properties.
By origin, combustible gases are divided into natural, or natural, and artificial, produced from solid and liquid fuels.
Natural gases are produced from wells of purely gas fields or oil fields along with oil. Gases from oil fields are called associated gases.
Gases from pure gas fields are mainly methane with a small content of heavy hydrocarbons. They are characterized by a constant composition and calorific value.
Associated gases, along with methane, contain a significant amount of heavy hydrocarbons (propane and butane). The composition and calorific value of these gases vary widely.
Artificial gases are produced at special gas plants - or are obtained as a by-product when coal is burned at metallurgical plants, as well as at oil refineries.
Gases produced from coal are used in our country for urban gas supply in very limited quantities, and their proportion is constantly decreasing. At the same time, the production and consumption of liquefied hydrocarbon gases, obtained from associated petroleum gases at gas-petrol plants and at oil refineries during oil refining, is growing. Liquefied petroleum gases used for urban gas supply are composed primarily of propane and butane.
Gas composition
The type of gas and its composition largely determine the field of gas application, the scheme and diameters of the gas network, the design solutions of gas burners and individual gas pipeline units.
Gas consumption depends on the calorific value, and hence the diameters of gas pipelines and the conditions for gas combustion. When gas is used in industrial installations, the combustion temperature and flame propagation speed and the constancy of the gas fuel composition are very important.The composition of gases, as well as their physicochemical properties, primarily depend on the type and method of obtaining gases.
Combustible gases are mechanical mixtures of various gases.<как горючих, так и негорючих.
The combustible part of gaseous fuel includes: hydrogen (H 2) -gas without color, taste or smell, its net calorific value is 2579 kcal / nm 3 \ methane (CH 4) is a colorless, tasteless and odorless gas that is the main combustible part of natural gases, its net calorific value is 8555 kcal / nm 3; carbon monoxide (CO) is a gas without color, taste or smell, it turns out due to incomplete combustion of any fuel, it is very poisonous, net calorific value 3018 kcal / nm 3; heavy-hydrocarbons (C p H t), By this name<и формулой обозначается целый ряд углеводородов (этан - С2Н 6 , пропан - С 3 Нв, бутан- С4Н 10 и др.), низшая теплотворная способность этих газов колеблется от 15226 до 34890 kcal / nm *.
The non-combustible part of the gaseous fuel includes: carbon dioxide (CO 2), oxygen (O 2) and nitrogen (N 2).
The non-combustible part of gases is usually called ballast. Natural gases are characterized by a high heating value and a complete absence of carbon monoxide. At the same time (a number of fields, mainly gas-oil fields, contain a very poisonous (and corrosively corrosive gas - hydrogen sulfide (H 2 S). Most artificial coal gases contain a significant amount of highly toxic gas - carbon monoxide (CO). The presence of oxide in the gas) carbon and other toxic substances are highly undesirable, since they complicate the production of operational work and increase the danger when using gas.In addition to the main components, the composition of gases includes various impurities, the specific value of which is negligible. even millions of cubic meters of gas, the total amount of impurities reaches a significant value. , and during operation.
The amount and composition of impurities depend on the method of production or extraction of gas and the degree of its purification. The most harmful impurities are dust, tar, naphthalene, moisture and sulfur compounds.
Dust appears in gas during production (extraction) or when transporting gas through pipelines. Tar is a product of thermal decomposition of fuel and is associated with many artificial gases. In the presence of dust in the gas, the resin contributes to the formation of tar-mud plugs and blockages of gas pipelines.
Naphthalene is commonly found in artificial coal gases. At low temperatures, naphthalene precipitates in pipes and, together with other solid and liquid impurities, reduces the flow area of gas pipelines.
Moisture in the form of vapor is found in almost all natural and artificial gases. It enters natural gases in the gas field itself as a result of gas contacts with the water surface, and artificial gases are saturated with water during the production process. The presence of moisture in the gas in significant quantities is undesirable, since it lowers the calorific value of the gas. , moisture during gas combustion carries away a significant amount of heat along with combustion products into the atmosphere. points) to be deleted. This requires the installation of special condensate traps and their evacuation.
Sulfur compounds, as already noted, include hydrogen sulfide, as well as carbon disulfide, mercaptan, etc. These compounds not only have a detrimental effect on human health, but also cause significant corrosion of pipes.
Among other harmful impurities, ammonia and cyanide compounds should be noted, which are found mainly in coal gases. The presence of ammonia and cyanide compounds leads to increased corrosion of the pipe metal.
The presence of carbon dioxide and nitrogen in combustible gases is also undesirable. These gases do not participate in the combustion process, being ballast that reduces the calorific value, which leads to an increase in the diameter of gas pipelines and to a decrease in the economic efficiency of using gaseous fuel.
The composition of gases used for city gas supply must meet the requirements of GOST 6542-50 (Table 1).
Table 1
The average values of the composition of natural gases of the most famous fields in the country are presented in table. 2.
From gas fields (dry)
| Western Ukraine. ... ... | 81,2 | 7,5 | 4,5 | 3,7 | 2,5 | - . | 0,1 | 0,5 | 0,735 | |
| Shebelinskoe ............................... | 92,9 | 4,5 | 0,8 | 0,6 | 0,6 | ____ . | 0,1 | 0,5 | 0,603 | |
| Stavropol region. ... | 98,6 | 0,4 | 0,14 | 0,06 | - | 0,1 | 0,7 | 0,561 | ||
| Krasnodar region. ... | 92,9 | 0,5 | - | 0,5 | _ | 0,01 | 0,09 | 0,595 | ||
| Saratov ............................... | 93,4 | 2,1 | 0,8 | 0,4 | 0,3 | Traces | 0,3 | 2,7 | 0,576 | |
| Gazli, Bukhara region | 96,7 | 0,35 | 0,4" | 0,1 | 0,45 | 0,575 | ||||
| From gas and oil fields (associated) | ||||||||||
| Romashkino ............................... | 18,5 | 6,2 | 4,7 | 0,1 | 11,5 | 1,07 | ||||
| 7,4 | 4,6 | ____ | Traces | 1,112 | __ . | |||||
| Tuymazy ............................... | 18,4 | 6,8 | 4,6 | ____ | 0,1 | 7,1 | 1,062 | - | ||
| Ash ....... | 23,5 | 9,3 | 3,5 | ____ | 0,2 | 4,5 | 1,132 | - | ||
| Fat .......... ............................. | 2,5 | . ___ . | 1,5 | 0,721 | - | |||||
| Syzran oil ............................... | 31,9 | 23,9 - | 5,9 | 2,7 | 0,8 | 1,7 | 1,6 | 31,5 | 0,932 | - |
| Ishimbay ............................... | 42,4 | 20,5 | 7,2 | 3,1 | 2,8 | 1,040 | _ | |||
| Andijan. ............................... | 66,5 | 16,6 | 9,4 | 3,1 | 3,1 | 0,03 | 0,2 | 4,17 | 0,801 ; | |
Calorific value of gases
The amount of heat released during the complete combustion of a unit of the amount of fuel is called the calorific value (Q) or, as they sometimes say, the calorific value, or calorific value, which is one of the main characteristics of the fuel.
The calorific value of gases is usually referred to 1 m 3, taken under normal conditions.
In technical calculations, normal conditions mean the state of the gas at a temperature equal to 0 ° C, and, at a pressure of 760 mmHg Art. The gas volume under these conditions is denoted nm 3(normal cubic meter).
For industrial gas measurements in accordance with GOST 2923-45, the normal conditions are taken as a temperature of 20 ° C and a Pressure of 760 mmHg Art. The volume of gas attributed to these conditions, in contrast to nm 3 will call m 3 (cubic meter).
Calorific value of gases (Q)) expressed in kcal / nm e or in kcal / m 3.
For liquefied gases, the calorific value is referred to 1 kg.
Distinguish between higher (Q in) and lower (Q n) calorific value. The gross calorific value takes into account the heat of condensation of water vapor generated during fuel combustion. The net calorific value does not take into account the heat contained in the water vapor of the combustion products, since water chests do not condense, but are carried away with the combustion products.
The concepts of Q in and Q n refer only to those gases, the combustion of which emits water vapor (these concepts do not apply to carbon monoxide, which does not produce water vapor during combustion).
During condensation of water vapor, heat is released, equal to 539 kcal / kg. In addition, when the condensate is cooled to 0 ° C (. Or 20 ° C), respectively, heat is released in the amount of 100 or 80 kcal / kg.
In total, more than 600 heat is released due to condensation of water vapor. kcal / kg, which is the difference between the gross and net calorific value of the gas. For most gases used in urban gas supply, this difference is 8-10%.
The calorific values of some gases are given in table. 3.
For urban gas supply, gases are currently used, which, as a rule, have a calorific value of at least 3500 kcal / nm 3. This is explained by the fact that in urban conditions gas is supplied through pipes over considerable distances. If the calorific value is low, a large amount must be fed. This inevitably leads to an increase in the diameters of gas pipelines and, as a consequence, to an increase in metal investments and funds for the construction of gas networks, and in the following: and to an increase in operating costs. A significant disadvantage of low-calorific gases is that in most cases they contain a significant amount of carbon monoxide, which increases the danger when using gas, as well as when servicing networks and installations.
Gas with a heating value of less than 3500 kcal / nm 3 most often used in industry, where it is not required to transport it over long distances and it is easier to organize incineration. For urban gas supply, it is desirable to have a constant calorific value. Fluctuations, as we have already established, are allowed no more than 10%. A large change in the calorific value of a gas requires a new adjustment, and sometimes a change in a large number of standardized burners for household appliances, which is associated with significant difficulties.
The heat of combustion is determined by the chemical composition of the combustible substance. Chemical elements contained in a combustible substance are indicated by accepted symbols WITH , N , O , N , S, and ash and water - symbols A and W respectively.
Collegiate YouTube
-
1 / 5
The heat of combustion can be referred to the working mass of the combustible substance Q P (\ displaystyle Q ^ (P)), that is, to the combustible substance in the form in which it comes to the consumer; to dry matter Q C (\ displaystyle Q ^ (C)); to the combustible mass of the substance Q Γ (\ displaystyle Q ^ (\ Gamma)), that is, to a combustible substance that does not contain moisture and ash.
Distinguish between the highest ( Q B (\ displaystyle Q_ (B))) and lower ( Q H (\ displaystyle Q_ (H))) heat of combustion.
Under higher calorific value understand the amount of heat that is released during the complete combustion of the substance, including the heat of condensation of water vapor when cooling the combustion products.
Net calorific value corresponds to the amount of heat that is released during complete combustion, excluding the heat of condensation of water vapor. The heat of condensation of water vapor is also called latent heat of vaporization (condensation).
The lowest and highest calorific values are related by the ratio: Q B = Q H + k (W + 9 H) (\ displaystyle Q_ (B) = Q_ (H) + k (W + 9H)),
where k is a coefficient equal to 25 kJ / kg (6 kcal / kg); W is the amount of water in the combustible substance,% (by weight); H is the amount of hydrogen in the combustible substance,% (by weight).
Calculation of the calorific value
Thus, the gross calorific value is the amount of heat released during the complete combustion of a unit mass or volume (for gas) of a combustible substance and cooling the combustion products to the dew point temperature. In thermal engineering calculations, the gross calorific value is taken as 100%. Latent heat of combustion of gas is the heat that is released during the condensation of water vapor contained in the combustion products. In theory, it can reach 11%.
In practice, it is not possible to cool the combustion products to complete condensation, and therefore the concept of the lowest heat of combustion (QHp) was introduced, which is obtained by subtracting from the highest heat of combustion the heat of vaporization of water vapor, both contained in the substance and formed during its combustion. The vaporization of 1 kg of water vapor consumes 2514 kJ / kg (600 kcal / kg). The net calorific value is determined by the formulas (kJ / kg or kcal / kg):
QHP = QBP - 2514 ⋅ ((9 HP + WP) / 100) (\ displaystyle Q_ (H) ^ (P) = Q_ (B) ^ (P) -2514 \ cdot ((9H ^ (P) + W ^ (P)) / 100))(for solid)
QHP = QBP - 600 ⋅ ((9 HP + WP) / 100) (\ displaystyle Q_ (H) ^ (P) = Q_ (B) ^ (P) -600 \ cdot ((9H ^ (P) + W ^ (P)) / 100))(for a liquid substance), where:
2514 - heat of vaporization at a temperature of 0 ° C and atmospheric pressure, kJ / kg;
H P (\ displaystyle H ^ (P)) and W P (\ displaystyle W ^ (P))- content of hydrogen and water vapor in working fuel,%;
9 is a coefficient showing that when 1 kg of hydrogen is burned in combination with oxygen, 9 kg of water are formed.
The heat of combustion is the most important characteristic of a fuel, as it determines the amount of heat obtained by burning 1 kg of solid or liquid fuel or 1 m³ of gaseous fuel in kJ / kg (kcal / kg). 1 kcal = 4.1868 or 4.19 kJ.
The net calorific value is determined experimentally for each substance and is a reference value. It can also be determined for solid and liquid materials, with a known elementary composition, by a calculation method in accordance with the formula of D. I. Mendeleev, kJ / kg or kcal / kg:
QHP = 339 ⋅ CP + 1256 ⋅ HP - 109 ⋅ (OP - SLP) - 25.14 ⋅ (9 ⋅ HP + WP) (\ displaystyle Q_ (H) ^ (P) = 339 \ cdot C ^ (P) +1256 \ cdot H ^ (P) -109 \ cdot (O ^ (P) -S_ (L) ^ (P)) - 25.14 \ cdot (9 \ cdot H ^ (P) + W ^ (P)))
QHP = 81 ⋅ CP + 246 ⋅ HP - 26 ⋅ (OP + SLP) - 6 ⋅ WP (\ displaystyle Q_ (H) ^ (P) = 81 \ cdot C ^ (P) +246 \ cdot H ^ (P) -26 \ cdot (O ^ (P) + S_ (L) ^ (P)) - 6 \ cdot W ^ (P)), where:
C P (\ displaystyle C_ (P)), H P (\ displaystyle H_ (P)), O P (\ displaystyle O_ (P)), S L P (\ displaystyle S_ (L) ^ (P)), W P (\ displaystyle W_ (P))- content of carbon, hydrogen, oxygen, volatile sulfur and moisture in the working mass of fuel in% (by mass).
For comparative calculations, the so-called conventional fuel is used, which has a specific heat of combustion equal to 29308 kJ / kg (7000 kcal / kg).
In Russia, thermal calculations (for example, the calculation of the heat load to determine the category of a room for explosion and fire hazard) is usually carried out according to the lowest heat of combustion, in the USA, Great Britain, France - according to the highest. In the United Kingdom and the United States, prior to the introduction of the metric system, calorific value was measured in British thermal units (BTU) per pound (lb) (1Btu / lb = 2.326 kJ / kg).
Substances and materials Net calorific value Q H P (\ displaystyle Q_ (H) ^ (P)), MJ / kg Petrol 41,87 Kerosene 43,54 Paper: books, magazines 13,4 Wood (bars W = 14%) 13,8 Natural rubber 44,73 Linoleum, polyvinyl chloride 14,31 Rubber 33,52 Staple fiber 13,8 Polyethylene 47,14 Expanded polystyrene 41,6 Loose cotton 15,7 Plastic 41,87 The tables show the mass specific heat of combustion of fuel (liquid, solid and gaseous) and some other combustible materials. The following fuels were considered: coal, firewood, coke, peat, kerosene, oil, alcohol, gasoline, natural gas, etc.
List of tables:
During an exothermic oxidation reaction of fuel, its chemical energy is converted into thermal energy with the release of a certain amount of heat. The resulting thermal energy is usually called the heat of combustion of the fuel. It depends on its chemical composition, humidity and is the main one. The heat of combustion of fuel per 1 kg of mass or 1 m 3 of volume forms the mass or volumetric specific heat of combustion.
Specific heat of combustion of fuel is the amount of heat released during the complete combustion of a unit of mass or volume of solid, liquid or gaseous fuel. In the International System of Units, this value is measured in J / kg or J / m 3.
The specific heat of combustion of the fuel can be determined experimentally or calculated analytically. Experimental methods for determining the calorific value are based on the practical measurement of the amount of heat released during the combustion of fuel, for example, in a calorimeter with a thermostat and a combustion bomb. For fuel with a known chemical composition, the specific heat of combustion can be determined using the Mendeleev formula.
Distinguish between higher and lower specific heats of combustion. The highest calorific value is equal to the maximum amount of heat released during the complete combustion of the fuel, taking into account the heat spent on the evaporation of moisture contained in the fuel. The lowest heat of combustion is less than the value of the highest one by the value of the heat of condensation, which is formed from the moisture of the fuel and the hydrogen of the organic mass, which is converted into water during combustion.
To determine fuel quality indicators, as well as in heat engineering calculations usually use the lowest specific heat of combustion, which is the most important thermal and performance characteristic of the fuel and is shown in the tables below.
Specific heat of combustion of solid fuel (coal, firewood, peat, coke)
The table shows the values of the specific heat of combustion of dry solid fuel in terms of MJ / kg. The fuel in the table is sorted alphabetically by name.
The highest calorific value of the considered solid fuels is possessed by coking coal - its specific heat of combustion is 36.3 MJ / kg (or in SI units 36.3 · 10 6 J / kg). In addition, high heat of combustion is characteristic of coal, anthracite, charcoal and lignite coal.
Fuels with low energy efficiency include wood, firewood, gunpowder, milling peat, oil shale. For example, the specific heat of combustion of firewood is 8.4 ... 12.5, and gunpowder - only 3.8 MJ / kg.
Specific heat of combustion of solid fuel (coal, firewood, peat, coke)
Fuel Anthracite 26,8…34,8 Wood pellets (pellets) 18,5 Dry firewood 8,4…11 Dry birch firewood 12,5 Gas coke 26,9 Blast furnace coke 30,4 Semi-coke 27,3 Powder 3,8 Slate 4,6…9 Combustible shale 5,9…15 Solid rocket fuel 4,2…10,5 Peat 16,3 Fibrous peat 21,8 Milling peat 8,1…10,5 Peat crumb 10,8 Brown coal 13…25 Brown coal (briquettes) 20,2 Brown coal (dust) 25 Donetsk coal 19,7…24 Charcoal 31,5…34,4 Hard coal 27 Coking coal 36,3 Kuznetsk coal 22,8…25,1 Chelyabinsk coal 12,8 Ekibastuz coal 16,7 Freztorf 8,1 Slag 27,5 Specific heat of combustion of liquid fuel (alcohol, gasoline, kerosene, oil)
The table of specific heats of combustion of liquid fuel and some other organic liquids is given. It should be noted that such fuels as gasoline, diesel fuel and oil are distinguished by high heat release during combustion.
The specific heat of combustion of alcohol and acetone is significantly lower than traditional motor fuels. In addition, liquid rocket fuel has a relatively low calorific value, and - with complete combustion of 1 kg of these hydrocarbons, an amount of heat equal to 9.2 and 13.3 MJ, respectively, will be released.
Specific heat of combustion of liquid fuel (alcohol, gasoline, kerosene, oil)
Fuel Specific heat of combustion, MJ / kg Acetone 31,4 Gasoline A-72 (GOST 2084-67) 44,2 Aviation gasoline B-70 (GOST 1012-72) 44,1 Gasoline AI-93 (GOST 2084-67) 43,6 Benzene 40,6 Diesel fuel winter (GOST 305-73) 43,6 Summer diesel fuel (GOST 305-73) 43,4 Liquid rocket fuel (kerosene + liquid oxygen) 9,2 Aviation kerosene 42,9 Lighting kerosene (GOST 4753-68) 43,7 Xylene 43,2 High-sulfur fuel oil 39 Low-sulfur fuel oil 40,5 Low-sulfur fuel oil 41,7 Sulphurous fuel oil 39,6 Methyl alcohol (methanol) 21,1 n-butyl alcohol 36,8 Oil 43,5…46 Methane oil 21,5 Toluene 40,9 White spirit (GOST 313452) 44 Ethylene glycol 13,3 Ethyl alcohol (ethanol) 30,6 Specific heat of combustion of gaseous fuel and combustible gases
The table of specific heats of combustion of gaseous fuel and some other combustible gases in terms of MJ / kg is presented. Of the gases considered, the largest mass specific heat of combustion differs. With the complete combustion of one kilogram of this gas, 119.83 MJ of heat will be released. Also, such a fuel as natural gas has a high calorific value - the specific heat of combustion of natural gas is 41 ... 49 MJ / kg (for a pure 50 MJ / kg).
Specific heat of combustion of gaseous fuel and combustible gases (hydrogen, natural gas, methane)
Fuel Specific heat of combustion, MJ / kg 1-Butene 45,3 Ammonia 18,6 Acetylene 48,3 Hydrogen 119,83 Hydrogen, mixture with methane (50% H 2 and 50% CH 4 by mass) 85 Hydrogen, mixture with methane and carbon monoxide (33-33-33% by mass) 60 Hydrogen mixed with carbon monoxide (50% H 2 50% CO 2 by mass) 65 Blast furnace gas 3 Coke oven gas 38,5 Liquefied petroleum gas (LPG) (propane-butane) 43,8 Isobutane 45,6 Methane 50 n-Bhutan 45,7 n-Hexane 45,1 n-Pentane 45,4 Associated gas 40,6…43 Natural gas 41…49 Propadien 46,3 Propane 46,3 Propylene 45,8 Propylene, mixture with hydrogen and carbon monoxide (90% -9% -1% by mass) 52 Ethane 47,5 Ethylene 47,2 Specific heat of combustion of some combustible materials
There is a table of specific heats of combustion of some combustible materials (wood, paper, plastic, straw, rubber, etc.). Of note are materials with high combustion heat. These materials include: rubber of various types, expanded polystyrene (foam), polypropylene and polyethylene.
Specific heat of combustion of some combustible materials
Fuel Specific heat of combustion, MJ / kg Paper 17,6 Leatherette 21,5 Wood (bars with a moisture content of 14%) 13,8 Wood in stacks 16,6 Oak wood 19,9 Spruce wood 20,3 The wood is green 6,3 Pine wood 20,9 Nylon 31,1 Carbolite products 26,9 Cardboard 16,5 Styrene-butadiene rubber SKS-30AR 43,9 Natural rubber 44,8 Synthetic rubber 40,2 SKS rubber 43,9 Chloroprene rubber 28 Linoleum, polyvinyl chloride 14,3 Two-layer polyvinyl chloride linoleum 17,9 Felt-based PVC linoleum 16,6 Linoleum, polyvinyl chloride on a warm basis 17,6 Linoleum, polyvinyl chloride on a fabric basis 20,3 Linoleum rubber (relin) 27,2 Paraffin wax 11,2 Polyfoam PVC-1 19,5 Styrofoam FS-7 24,4 Foam FF 31,4 Expanded polystyrene PSB-S 41,6 Polyurethane foam 24,3 Fiber board 20,9 Polyvinyl chloride (PVC) 20,7 Polycarbonate 31 Polypropylene 45,7 Polystyrene 39 High pressure polyethylene 47 Low-pressure polyethylene 46,7 Rubber 33,5 Roofing material 29,5 Channel soot 28,3 Hay 16,7 Straw 17 Organic glass (plexiglass) 27,7 Textolite 20,9 Tol 16 TNT 15 Cotton 17,5 Cellulose 16,4 Wool and wool fibers 23,1 Sources:
- GOST 147-2013 Solid mineral fuel. Determination of gross calorific value and calculation of net calorific value.
- GOST 21261-91 Petroleum products. Method for determining the gross calorific value and calculating the net calorific value.
- GOST 22667-82 Natural combustible gases. Calculation method for determining the calorific value, relative density and Wobbe number.
- GOST 31369-2008 Natural gas. Calculation of calorific value, density, relative density and Wobbe number based on component composition.
- Zemskiy G.T.
Gas fuel is divided into natural and artificial and is a mixture of combustible and non-combustible gases, containing a certain amount of water vapor, and sometimes dust and tar. The amount of gas fuel is expressed in cubic meters under normal conditions (760 mm Hg and 0 ° C), and the composition is expressed as a percentage by volume. The composition of the fuel is understood as the composition of its dry gaseous part.
Natural gas fuel
The most common gas fuel is natural gas, which has a high calorific value. The basis of natural gas is methane, the content of which is 76.7-98%. Other gaseous hydrocarbon compounds are found in natural gas from 0.1 to 4.5%.
Liquefied gas is a product of oil refining - it consists mainly of a mixture of propane and butane.
Natural gas (CNG, NG): methane CH4 over 90%, ethane C2 H5 less than 4%, propane C3 H8 less than 1%
Liquefied gas (LPG): propane C3 H8 more than 65%, butane C4 H10 less than 35%
The composition of combustible gases includes: hydrogen H 2, methane CH 4, Other hydrocarbon compounds C m H n, hydrogen sulfide H 2 S and non-combustible gases, carbon dioxide CO2, oxygen O 2, nitrogen N 2 and a small amount of water vapor H 2 O. Indexes m and NS at C and H characterize compounds of various hydrocarbons, for example, for methane CH 4 t = 1 and n= 4, for ethane C 2 H b t = 2 and n= b, etc.
Dry gaseous fuel composition (percent by volume):
CO + H 2 + 2 C m H n + H 2 S + CO 2 + O 2 + N 2 = 100%.The non-combustible part of dry gas fuel - ballast - is nitrogen N and carbon dioxide CO 2.
The composition of the wet gaseous fuel is expressed as follows:
CO + H 2 + Σ C m H n + H 2 S + CO 2 + O 2 + N 2 + H 2 O = 100%.
The heat of combustion, kJ / m (kcal / m 3), 1 m 3 of pure dry gas under normal conditions is determined as follows:
Q n c = 0.01,
where Qco, Q n 2, Q s m n n Q n 2 s. - heat of combustion of individual gases included in the mixture, kJ / m 3 (kcal / m 3); CO, H 2, Cm H n, H 2 S - components that make up the gas mixture,% by volume.
The heat of combustion of 1 m3 of dry natural gas under normal conditions for most domestic fields is 33.29 - 35.87 MJ / m3 (7946 - 8560 kcal / m3). The characteristics of gaseous fuels are shown in table 1.
Example. Determine the net calorific value of natural gas (under normal conditions) of the following composition:
H 2 S = 1%; CH 4 = 76.7%; C 2 H 6 = 4.5%; C 3 H 8 = 1.7%; C 4 H 10 = 0.8%; C 5 H 12 = 0.6%.
Substituting the characteristics of gases from Table 1 into formula (26), we get:
Q ns = 0.01 = 33981 kJ / m 3 or
Q ns = 0.01 (5585.1 + 8555 76.7 + 15 226 4.5 + 21 795 1.7 + 28 338 0.8 + 34 890 0.6) = 8109 kcal / m 3.
Table 1. Characteristics of gaseous fuels
Gas
Designation
Heat of combustion Q n s
KJ / m3
Kcal / m3
Hydrogen H, 10820 2579 Carbon monoxide CO 12640 3018 Hydrogen sulfide H 2 S 23450 5585 Methane CH 4 35850 8555 Ethane C 2 H 6 63 850 15226 Propane C 3 H 8 91300 21795 Butane C 4 H 10 118700 22338 Pentane C 5 H 12 146200 34890 Ethylene C 2 H 4 59200 14107 Propylene C 3 H 6 85980 20541 Butylene C 4 H 8 113 400 27111 Benzene C 6 H 6 140400 33528 DE boilers consume from 71 to 75 m3 of natural gas to produce one ton of steam. Gas cost in Russia for September 2008 is 2.44 rubles per cubic meter. Consequently, a ton of steam will cost 71 × 2.44 = 173 rubles 24 kopecks. The real cost of a ton of steam at factories is at least 189 rubles per ton of steam for DE boilers.
DKVR boilers consume from 103 to 118 m3 of natural gas to produce one ton of steam. The minimum estimated cost of a ton of steam for these boilers is 103 × 2.44 = 251 rubles 32 kopecks. The real cost of steam for factories is at least 290 rubles per ton.
How to calculate the maximum consumption of natural gas for a DE-25 steam boiler? These are the technical characteristics of the boiler. 1840 cubes per hour. But you can calculate. 25 tons (25 thousand kg) must be multiplied by the difference between the enthalpies of steam and water (666.9-105) and all this must be divided into the boiler efficiency of 92.8% and the heat of combustion of the gas. 8300. and all
Artificial gas fuel
Artificial combustible gases are local fuels, since they have a significantly lower calorific value. Their main fuel elements are carbon monoxide CO and hydrogen H2. These gases are used within the production, where they are produced as a fuel for technological and power plants.
All natural and artificial combustible gases are explosive and can ignite on an open fire or spark. A distinction is made between the lower and upper explosive limits of a gas, i.e. the highest and lowest percentage of its concentration in the air. The lower explosive limit of natural gases ranges from 3% to 6%, and the upper limit - from 12% to 16%. All flammable gases can poison the human body. The main toxic substances of combustible gases are: carbon monoxide CO, hydrogen sulfide H2S, ammonia NH3.
Natural combustible gases, as well as artificial ones, are colorless (invisible), odorless, which makes them dangerous when they penetrate into the interior of the boiler room through leaks in the gas pipeline fittings. To avoid poisoning, flammable gases should be treated with an odorless odorant.
Obtaining carbon monoxide CO in industry by gasification of solid fuel
For industrial purposes, carbon monoxide is obtained by gasifying solid fuel, i.e. converting it into gaseous fuel. So you can get carbon monoxide from any solid fuel - fossil coal, peat, firewood, etc.
The process of gasification of solid fuel is shown in a laboratory experiment (Fig. 1). Having filled the refractory tube with pieces of charcoal, we will heat it up strongly and let oxygen pass from the gasometer. Let the gases coming out of the tube pass through a wash bottle with lime water and then ignite. Lime water becomes turbid, the gas burns with a bluish flame. This indicates the presence of CO2 dioxide and carbon monoxide CO in the reaction products.
The formation of these substances can be explained by the fact that when oxygen comes into contact with hot coal, the latter is first oxidized to carbon dioxide: C + O 2 = CO 2
Then, passing through hot coal, carbon dioxide is partially reduced by it to carbon monoxide: CO 2 + C = 2CO
Rice. 1. Obtaining carbon monoxide (laboratory experiment).
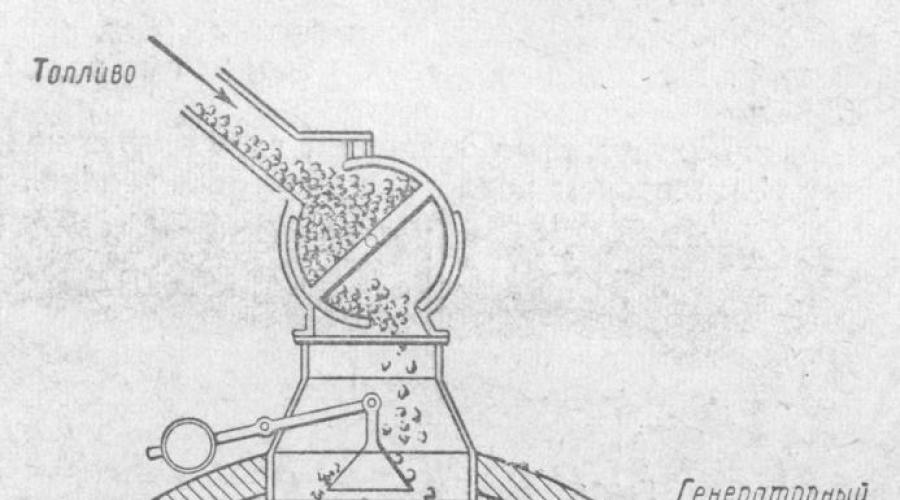
Under industrial conditions, solid fuel gasification is carried out in furnaces called gas generators.
The resulting gas mixture is called producer gas.
The gas generator device is shown in the figure. It is a steel cylinder with a height of about 5 m and a diameter of about 3.5 m, lined inside with refractory bricks. The gas generator is loaded with fuel from above; from below, through the grate, air or water vapor is supplied by a fan.
Oxygen in the air reacts with carbon in the fuel, forming carbon dioxide, which, rising up through the hot fuel bed, is reduced by carbon to carbon monoxide.
If only air is blown into the generator, then a gas is obtained, which in its composition contains carbon monoxide and nitrogen in the air (as well as a certain amount of CO 2 and other impurities). This generator gas is called air gas.
If water vapor is blown into the generator with hot coal, then as a result of the reaction, carbon monoxide and hydrogen are formed: C + H 2 O = CO + H 2
This gas mixture is called water gas. Water gas has a higher calorific value than air gas, since, along with carbon monoxide, it also contains a second combustible gas - hydrogen. Water gas (synthesis gas), one of the products of gasification of fuels. Water gas consists mainly of CO (40%) and H2 (50%). Water gas is a fuel (calorific value of 10,500 kJ / m3, or 2,730 kcal / mg) and at the same time a raw material for the synthesis of methyl alcohol. Water gas, however, cannot be produced for a long time, since the reaction of its formation is endothermic (with heat absorption), and therefore the fuel in the generator cools down. To keep the coal glowing, the injection of water vapor into the generator is alternated with the injection of air, the oxygen of which is known to react with the fuel to generate heat.
Recently, steam-oxygen blast has been widely used for fuel gasification. The simultaneous blowing of water vapor and oxygen through the fuel bed allows the process to be carried out continuously, to significantly increase the generator's productivity and to obtain gas with a high content of hydrogen and carbon monoxide.
Modern gas generators are powerful continuous devices.
To prevent flammable and poisonous gases from entering the atmosphere when fuel is supplied to the gas generator, the loading drum is made double. While fuel enters one compartment of the drum, the other compartment spills fuel into the generator; when the drum rotates, these processes are repeated, while the generator remains isolated from the atmosphere all the time. Uniform distribution of fuel in the generator is carried out by means of a cone, which can be installed at different heights. When it is lowered, the coal lays down closer to the center of the generator, when the cone is raised, the coal is thrown closer to the walls of the generator.
Ash removal from the gas generator is mechanized. The cone-shaped grate is slowly rotated by an electric motor. In this case, the ash is displaced to the walls of the generator and, by special devices, is dumped into the ash box, from where it is periodically removed.
The first gas lanterns were lit in St. Petersburg on Aptekarsky Island in 1819. The gas that was used was obtained by gasification of coal. It was called lamp gas.
The great Russian scientist D.I.Mendeleev (1834-1907) was the first to express the idea that coal gasification can be carried out directly underground without lifting it out. The tsarist government did not appreciate this proposal of Mendeleev.
The idea of underground gasification was warmly supported by V. I. Lenin. He called it "one of the great victories of technology." Underground gasification was carried out for the first time by the Soviet state. Already before the Great Patriotic War, underground generators in the Donetsk and Moscow region coal basins worked in the Soviet Union.
An idea of one of the methods of underground gasification is given in Figure 3. Two wells are laid into the coal seam, which are connected at the bottom with a channel. Coal is ignited in such a channel near one of the wells and blown is supplied there. Combustion products, moving along the channel, interact with hot coal, as a result of which a combustible gas is formed, as in a conventional generator. The gas comes out to the surface through the second well.
Generator gas is widely used to heat industrial furnaces - metallurgical, coke oven and as fuel in cars (Fig. 4).
Rice. 3. Scheme of underground coal gasification.
A number of organic products are synthesized from hydrogen and carbon monoxide of water gas, for example, liquid fuel. Synthetic liquid fuel - fuel (mainly gasoline) obtained by synthesis from carbon monoxide and hydrogen at 150-170 g Celsius and a pressure of 0.7 - 20 MN / m2 (200 kgf / cm2), in the presence of a catalyst (nickel, iron, cobalt ). The first production of synthetic liquid fuels was organized in Germany during the 2nd World War due to a shortage of oil. Synthetic liquid fuel has not become widespread due to its high cost. Water gas is used to produce hydrogen. For this, water gas mixed with water vapor is heated in the presence of a catalyst and as a result, hydrogen is obtained in addition to that already present in the water gas: CO + H 2 O = CO 2 + H 2