Characteristics of the oceanic water medium. Salinity
The land can be called a water planet with confidence, because the world ocean surrounding the land covers 71% of its entire surface. included in its composition, differ in many ways from each other. Including such a parameter as salinity meaning the number of salts dissolved in one liter of water under certain conditions. The salinity of seawater is measured most often in "‰" (PROMIL). Now it will be not difficult to find out what the salted sea on the ground.
5. The Ionian Sea - salinity exceeds 38
The Ionian Sea is called part of the Mediterranean, which is washes the shores of southern Italy and Greece. The bottom of the sea is covered with sludge, and closer to the shores - sand and small shell. Its area is 169 thousand km², the maximum depth is 5 121 m. It is the largest depth on the entire Mediterranean. Industrial collections of mackerel, Kefali, Tuna, Cambals are underway. The water of the Ionian Sea safe and very warm, even in February their temperature does not fall below 14 ° C, and in the peak of the holiday season, in August, reaches 25.5 ° C. Among its inhabitants can be called dolphins aphalines, huge turtles, octopus. And very dangerous marine heels and white sharks almost can not be found near the coast. Poisonous fish drainers who can cause an allergic response in humans are more active at night, in the afternoon buried in the sand.
4. Aegean Sea - salinity from 37 to 40.0
This semi-closed sea has about 20,000 islands and is located in the eastern part of the Mediterranean. Total area - 179 thousand km². Through the straits is connected to marble, black and Mediterranean seas. The salinity indicators of its waters increase that they are associated with universal warming. After bathing, it is recommended to wash off sea water, as it can adversely affect the skin condition and the mucous membrane of the eyes. In the Aegean Sea there is fisheries, the sponges are actively producing, they catch octopus. Due to the fact that this sea is little plankton, fisheries in its waters gradually falls into decline.
3. Ligurian Sea - Salon 38
This sea is located in the western part of the Mediterranean. The shores are incisive and rocky, but there are sandy beaches. In the Ligurian Sea, there are many small rivers, which originate in Apennines. On its shores there are important ports such as:
- Lympia, which is considered the sea gate of Nice.
- Savon cruise ports, spice having container and bulk terminals.
- The Genoese Port, which takes the first place in the trade volume in Italy.
Despite the high salinity of these waters, on the French-Italian coast of the Ligurian Sea is one of the most famous resort areas in the world - Riviera.
2. Mediterranean Sea - salinity from 36 to 39.5 ‰
The Mediterranean Sea is the relic of the ancient Ocean Tetis. It is considered one of the largest in size by sea, its area is 2.5 million km². His pool includes Azov, black and marble seas. The salinity of the sea fluctuates significantly, as water comes from the Atlantic through the Gibraltar Strait, the salinity of which is much lower. The amount of zooplankton in the Mediterranean Sea is relatively small, as a result of this, a little and various species of fish, as well as marine animals and mammals. But in large quantities are algae, especially Peridine and diatoms. The bottom fauna is very poor because of a yellowish alley, which does not favor the development of life. Fish in the Mediterranean Sea - 550 species, of which 70 are endemics. More often than others are: mackerel, sardine, stavride, kefal, etc. There are larger "residents" - sharks, races, tuna. Sound mollusks are common.
1. Red Sea - salinity 41
The very saline of all, the Red Sea is in the tectonic depression, the depth of which can reach up to 3 km. It is the inner sea of \u200b\u200bthe Indian Ocean. Hot climate, provoking a strong surface evaporation and a small amount of precipitation (approximately 100 mm per year), the absence of rivers flowing into the sea leads to a gradual increase in its salinity. Due to the lack of sludge and sand, which is much in river water, the Red Sea is characterized by extraordinary transparency and purity. The water temperature even in winter is +20 ° C, and in the summer - much higher.
Despite its salinity, the water of the Red Sea is amazed by a huge amount of fish living in it. But ichthyologists believe that only 60% of fish are openly capable of exist at great depth. The sea is unusually beautiful, and there are many interesting and sometimes funny inhabitants in it, but they are categorically prohibited to touch them. Corals, sponges, jellyfish, as well as marine hedgehogs, moray and poisonous sea snakes are potentially extremely dangerous. Any contact with them may end with a burn, significant blood loss or a sharply expressed allergic reaction, and sometimes with a fatal outcome. In warm sea waters, 44 types of sharks live. The most terrible of them is a tiger, which can easily attack a person.
Having considered separately, it is now easy to conclude what the salt sea is. The salinity of the very famous Dead Sea comes with 350, but in fact, despite the name, it is a light lake, which gradually dries.
The fact that water in the sea salty - everyone knows no obstacle. But answer the question of which the sea is the most salty on the planet, most people are most likely difficult. However, it was unlikely that a person was thinking about why the sea is salty and is there a life in the world in the world in the world.
The world ocean is a single whole natural organism. On the planet, he takes two thirds of the whole earthly space. Well, marine water, which fills the world ocean, is considered the most common substance on the surface of the Earth. She has a bitter-salty taste, from fresh water, sea is distinguished by transparency and color, specific weight and aggressive impact on materials. But this is simply explained - in seawater there are more than 50 different components.
Most Salted Sea World
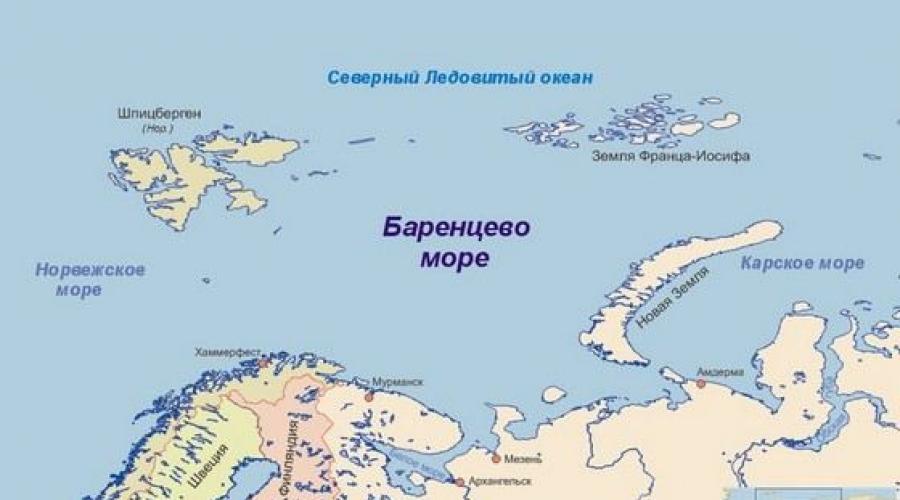
Which seas are more salty, what less - scientists know for sure. The liquid in the seas has already been studied and literally decomposed on the component. And it turned out that the salty seas in Russia occupy the highest lines in the ranking of salinity. So, the main challenger on the status of the salty itself is the Barents Sea. All because during the year the salinity of the surface layers fluctuates in the area of \u200b\u200b34.7-35 percent, however, if deviate to the north and east, the percentage will decrease. 
The white sea is also distinguished by high salinity. In the surface layers, the indicator stopped at 26 percent, but at a depth it increases to 31 percent. In the Kara Sea salinity about 34 percent, however, it is heterogeneous and in the mouths of the flowing rivers water becomes almost fresh. Another of the most salty seas of the world can be called Laptev Sea. The surface records salinity at 28 percent. An even greater indicator is 31-33 percent - in the Chukchi Sea. But it is in winter, in the summer salinity descends.

What kind of sea salty
By the way, all the favorite Mediterranean Sea can also compete for the status of the most saline in the world. Saltness in it varies from 36 to 39.5 percent. In particular, because of this, the sea is noted a weak quantitative development of phyto and zooplankton. However, despite this, a large number of fauna representatives live in the sea. Here you can meet seals, sea turtles, 550 species of fish, about 70 fish endemics, cancer, as well as octopus, crabs, lobs, squid. 
It is certainly not a salty of the Mediterranean another famous sea - the Caspian Sea. Caspian boasts a rich animal world - 1809 species. Most of the world stocks of sturgeon live in the sea, as well as freshwater fish (Sudak, Sazan and Vobla). The plant world is also very rich - in the Caspian Sea 728 species of plants, but prevail, of course, algae. An interesting fact, in Karakalpakstan there is a unique natural object - the Aral Sea. And his distinctive feature is that it can be called the second Dead Sea. Another half a century ago, the Aral Sea had a standard salinity. However, as soon as the water from the sea began to take land for irrigation, the salinity began to rise, and by 2010 it raised 10 times. The dead sea is called not only in salinity indicators, but also due to the fact that many inhabitants of the Aral Sea as a protest against raising the level of salinity extinct.
Why salty seas
Why salty seas - this question was interested in people for a long time. For example, according to the Norwegian legend at the bottom of the seas, an unusual mill is standing, which is constantly challenged salt. Similar stories are in the fairy tales of residents of Japan, Philippines and Karelia. But in the Crimean legend, the Black Sea is salty due to the fact that the girls who have fallen in Neptune's network are forced to weave white lace for the waves and constantly crying their native land. From tears water and became salty. 
But according to scientific hypothesis, salted water has become different. All water in the seas and oceans is taken from rivers. However, fresh water flows in the latter. And on average, 35 grams of salts was dissolved in one liter of the world's ocean. According to scientists, each tip of salt is washed by river waters from the soil and goes to the sea. Over the ages and millennia of salt in the world ocean, more and more are intertwined. And she can not leave anywhere.

There is a version that water in the oceans and the seas initially was salted. The first reservoir on the planet, allegedly, was filled with acid rain, which fell to the ground as a result of a major eruption of volcanoes at the beginning of the life of the planet. Acid, according to scientists, corrosion rocks, entered them into chemical compounds. As a result of chemical reactions, salted water appeared, which now fills the world ocean.
Most Salted Sea in the world
The very saline sea in the world is called red. In one liter of its water contains 41 grams of salts. By the sea there is only one source of water intake - the Aden Bay. During the year through the Bab El Mongland, the Red Sea receives a thousand cubic kilometers of water more than being taken out of the sea. Therefore, according to researchers, the Water of the Red Sea is fully updated, it is necessary for about 15 years. 
The salty red sea is very well and evenly mixed. In winter, surface waters are cooled, lowered down, lifting warm water from the sea depth. In the summer, the water from the surface evaporates, the remaining becomes salty and heavy, so it sinks down. Ups up not so salted water. Thus, water and mixed. The sea is equally in salinity and temperatures everywhere, except for depression.
By the way, the detection of the depression in the Red Sea with hot brine in the 60s of the last century was a present discovery for scientists .. brine in such depressions has a temperature of 30 to 60 degrees Celsius, and it increases a maximum of 0.7 degrees per year. It turns out that water is heated from the inside "earth" heat. And scientists declare that the brine does not mix with sea water and differs from it by chemical indicators.

In the Red Sea, there is a completely lack of coastal stock (rivers and rainflows). As a result, there is no dirt from sushi, but there is crystal transparency of water. All year round, the temperature is held at 20-25 degrees. This has led wealth, as well as the uniqueness of the marine life in the sea.
Why the Red Sea is the most salty? Some say the most salty is the Dead Sea. Its salinity is 40 times higher than the salinity of the Baltic Sea and 8 times the Atlantic Ocean. However, to call the Dead Sea is the most salty - it is impossible, but it is considered the warmest.
The Dead Sea is located in Jordan and Israel in West Asia. Its area is more than 605 square kilometers at a maximum depth of 306 meters. In this famous Sea falls the only river - Jordan. There is no exit by the sea, so it is more correct to call it more correctly by the lake.
Subscribe to our channel in Yandex.Dzen
3. Characteristics of the oceanic water medium.
© Vladimir Kalanov,
"Knowledge is power".
Oceanic medium, that is, sea water is not just known to us from the very birth agent, which is hydrogen oxide H 2 O. Sea water is a solution of a wide variety of substances. In the waters of the World Ocean are almost all known chemical elements in the form of various compounds.
Most of all in seawater dissolved chlorides (88.7%), among which the sodium chloride prevails, that is, an ordinary salt salt NaCl. Significantly less is contained in seawater sulfates, that is, sulfuric acid salts (10.8%). All other substances account for only 0.5% of the total salt composition of seawater.
After sodium salts in second place in seawater, magnesium salts are standing. This metal is used in the manufacture of lungs and durable alloys necessary in mechanical engineering, especially in aircraft construction. Each cubic meter of seawater contains 1.3 kilograms of magnesium. The technology of its extraction from sea water is based on the transfer of its soluble salts into insoluble compounds and the deposition of their lime. The cost of magnesium produced directly from sea water turned out to be significantly lower than the cost of this metal, which was previously mined from ore materials, in particular, dolomites.
It is worth noting that the French chemist opened in 1826 A. Balyar Brom is not contained in any mineral. You can get broma only from seawater, where it is contained in a relatively small amount - 65 grams per cubic meter. Bromine is used in medicine as a soothing agent, as well as in photographs and petrochemistry.
Already at the end of the XX century, the ocean began to give 90% of world bromine production and 60% magnesium. Sodium and chlorine is produced from sea water in significant quantities. As for the food (cooking) salt, the man has long received it from seawater by evaporation. Marine salt fishery still act in tropical countries, where the salt is obtained right in shallow segments, burning them off with dams from the sea. The technology is not very complicated here. The concentration of the cook salt in water is higher than the remaining salts, and therefore, when evaporated, it falls precipitated. The crystals at the bottom of the crystals are removed from the so-called mother liquor and washed with fresh water to remove the residues of magnesium salts, which give salts of bitter taste.
The more advanced production of seawater salt production is used on numerous salvers of France and Spain, which in large volumes supply salt not only to the European market. For example, one of the new ways to produce salt is that special seasons of seawater are installed in the basins of seawren. Water, turned into dust (suspension), has a huge area of \u200b\u200bevaporation and from the smallest droplets, it evaporates instantly, and only salt falls on the ground.
The extraction of the cooking salt from sea water will increase in the future, because the deposits of the stone salt, as well as other minerals, sooner or later deplete. Currently, about a quarter of all the necessary humanity of the cooking salt is mined in the sea, the rest of the amount is mined in salt cops.
This contains in seawater also iodine. But the process of obtaining iodine directly from the water would be completely unprofitable. Therefore, iodine is obtained from dried brown algae growing in the ocean.
Even gold is contained in ocean water, though in insignificant quantities - 0.00001 grams per cubic meter. Known attempt by German chemists in the 1930s to extract gold from the waters of the German Sea (so in German is often called the North Sea). However, filled the repository of Reichsbank with gold ingots failed: production costs would exceed the cost of gold itself.
Some scientists suggest that in the next few decades it can be economically appropriate to obtain from the sea of \u200b\u200bheavy hydrogen (deuterium), and then humanity will be provided with energy to millions of years ahead ... But the uranium from sea water is already produced on an industrial scale. Since 1986, the world's first plant for extracting uranium from sea water is operating on the shores of the Inner Japanese Sea. A complex and expensive technology is designed to receive 10 kg of metal per year. To obtain such a quantity of uranium, more than 13 million tons of seawater is required to be filtered and subjected to ionic treatment. But the Japanese persistent in labor cope with this work. In addition, they are well known what atomic energy is. -)
An indicator of the amount of chemicals dissolved in water is a special characteristic, which is called saline. Saltness is a mass of all salts in grams contained in 1 kg of sea water. Saltivity is measured in thousands of thousands, or PROMILL (‰). On the surface of the open ocean, the saline oscillations are small: from 32 to 38. The average surface salinity of the world ocean is about 35 ‰ (more accurately - 34.73).

The water of the Atlantic and Pacific Oceans has salting just above the average value (34.87 ‰), and the water of the Indian Ocean is slightly lower (34.58 ‰). It affects the distinguishing effect of Antarctic ice. For comparison, we indicate that the usual solidity of river water does not exceed 0.15, which is 230 times less than the surface saline of sea water.
The least salty in the open ocean are the water of the indoor districts of both hemispheres. This is explained by the melting of continental ice, especially in the southern hemisphere, and large volumes of river wastewater in the northern hemisphere.
Maldness increases to the tropics. The largest concentration of salts is observed not at the equator, but in the latitude of 3 ° -20 ° to the south and north of the equator. These bands are sometimes called salting belts.
The fact that in the equatorial zone the surface saltness of water is relatively low, is explained by the fact that the equator is a zone of pouring tropical rains that are clearing water. Often in the area of \u200b\u200bthe equator, dense clouds close the ocean from direct sunlight, which reduces water evaporation at such moments.
In the outskirts and especially in the inner seas, salting differs from the ocean. For example, in the Red Sea, the surface saltness of water reaches the highest in the ocean of values \u200b\u200b- to 42. It is simply explained: the Red Sea is located in the high evaporation zone, and with the ocean it is reported through the shallow and non-shimmer of Bab el-Mandant, and there is no freshwater from the continent, since it does not fall into this sea, and rare rains It is not able to disperse the water noticeably.
The Baltic Sea, far-out of sushi, communicates with the ocean through several small and narrow straits, is located in the zone of moderate climate and takes the water of many large rivers and small rivers. Therefore, Baltika is one of the most disperse pools of the World Ocean. The surface salinity of the central part of the Baltic Sea is only 6-8, and in the north, even up to 2-3 ‰) is descended in the shallow boots.
With an increase in the depth of saline changes. This is explained by the movement of subsurface water, that is, the hydrological regime of a particular pool. For example, in the equatorial latitudes of the Atlantic and Pacific Oceans below 100-150 m, layers of very salted waters are traced (above 36 ‰), which are formed due to the depth anti-countercurrents from Western outskins of the oceans of more saline tropical waters.
Saltance changes sharply only to depths of about 1500 m. Below this horizon of salting oscillation is almost not observed. At high depths of different oceans, salting rates come closer. Seasonal changes in saline on the surface of the open ocean are insignificant, not more than 1.
Saltness anomalies Experts consider the saltness of water in the Red Sea at a depth of about 2000 m, which reaches 300.
The main method of determining the saline of sea water is the titration method. The essence of the method is that a certain amount of silver nitric acid (AGNO 3) is added to the water sample (AGNO 3), which, in compound with chloride, seawater sodium falls into a precipitate in the form of silver chloride. Since the ratio of the amount of sodium chloride to other substances dissolved in the water is constantly, then by weighing the precipitated silver chloride, it is possible to simply just calculate the saltness of the water.
There are other ways to define salting. Since such, for example, indicators, as the refraction of light in water, the density and electrical conductivity of water depend on its saline, then, by determining them, the saline of water can be measured.
Take samples of sea water to determine its saline or other indicators - a very difficult thing. For this, they use special sampler - by trampling, providing samples from different depths or from different water layers. This process requires a lot of attention and caution from hydrolyologists.
So, the main processes affecting the saltness of water are the rate of water evaporation, the intensity of the mixing of more salted waters with less salted, as well as the frequency and intensity of precipitation. These processes are determined by the climatic conditions of this or that area of \u200b\u200bthe World Ocean.
In addition to these processes, the intimacy of melting glaciers and freshwater volumes, brought by rivers affect the salty of sea water.
In general, the percentage of various salts in sea water in all parts of the ocean almost always remains the same. However, in separate places on the chemical composition of seawater, marine organisms are noticeable. They are used for their power and development, many substances dissolved in the sea, although in various quantities. Some substances, such as phosphates and nitrogen compounds, are consumed especially in large volumes. In areas where marine organisms are many, the content of these substances in water is somewhat decreased. A noticeable effect on the chemical processes occurring in seawater, the smallest organisms are included in the plankton. They drift on the surface of the sea or in the near-surface water layers and, dying, slowly and continuously fall on the bottom of the ocean.

Malty of the World Ocean. Map of current monitoring (Enlarge).
What is the general content of salts in the World Ocean? Now it's not difficult to answer this question. If you proceed from the fact that the total amount of water in the world ocean is 1370 million cubic kilometers, and the average salt concentration in sea water is 35 ‰, that is, 35 g in one liter, it turns out that in one cubic meter contains approximately 35 thousand tons. Salt. Then the amount of salt in the world ocean will express the astronomical digit of 4.8 * 10 16 tons (that is, 48 \u200b\u200bquadrillion tons).
This means that even active extraction of salts for household and industrial needs will not be able to change the composition of sea water. In this regard, the ocean without exaggeration can be considered inexhaustible.
Now it is necessary to answer for an equally important question: where in the ocean so much salt?
For many years, the hypothesis was dominant in science that rivers brought salt into the sea. But this hypothesis, at first glance, quite convincing, turned out to be scientifically insolven. It has been established that every second of the river of our planet is in the ocean about a million tons of water, and their annual stock is equal to 37 thousand cubic kilometers. For a complete renewal of water in the world ocean, 37 thousand years is required - approximately this time can be river flow to fill the ocean. And if we assume that there were no less than one hundred thousand such periods in the geological history of the land, and the content of salt in river water in the average approximation is about 1 gram per liter, it turns out that for the entire geological history of land in the ocean rivers were made about 1, 4 * 10 20 tons of salts. And according to the calculation of scientists, which we have just led, 4.8 * 10 16 tons of salt dissolved in the world ocean, that is, 3 thousand times less. But it's not only that. The chemical composition of salts dissolved in river water is sharply different from the composition of the sea salt. If in the sea water, sodium and magnesium compounds with chlorine (89% of the dry residue after evaporation of water and only 0.3% is carbonated calcium), then in river water carbon dioxide ranks first - over 60% of dry residue, and sodium chlorides And magnesium together - only 5.2 percent.
Scientists have one assumption left: the ocean became salty in the process of his birth. The most ancient animals could not exist in weakly fat, and even more so in fresh pools. It means that the composition of sea water did not change since its inception. But where were the carbonates coming to the ocean, along with river runoff for hundreds of millions of years? The only correct answer to this question was the founder of Biogeochemistry, the Great Russian Academician V.I. Vernadsky. He argued that almost all carbon dioxide, as well as salts of silicon, brought by rivers in the ocean, are immediately removed from the solution of the sea plants and animals, which these minerals are needed for their skeletons, shells and shells. As these living organisms, the carbon dioxide (CaCO 3) and silicon salts are deposited on the seabed in the form of organic origin, as eliminating these living organisms. So living organisms throughout the entire time of the existence of the world's ocean support the constant composition of its salts.
And now a few words are about one mineral contained in sea water. We spent so many words to praise the ocean because it contains many different salts and other substances, including such as deuterium, uranium and even gold. But we did not mention the main and main mineral, which is located in the World Ocean - about simple water H 2 O. . Without this "mineral" on Earth, there would be nothing at all: neither the oceans or the seas or us with us. We had the opportunity to talk about the basic physical properties of water. Therefore, here we will limit ourselves to some comments.
In the entire history of science, people did not solve all the secrets of this fairly simple chemical, whose molecule consists of three atoms: two hydrogen atoms and one oxygen atom. By the way, modern science claims that hydrogen atoms make up 93% of all atoms of the universe.
And among the mysteries and secrets of water, for example, such: why frozen water pairs turn into snowflakes, the shape of which is a surprisingly correct geometric figure resembling magnificent patterns. And drawings on window windows in frosty days? Instead of amorphous snow and ice, we see crystalline ice, which lined up so amazingly that they look like the leaves and branches of some fabulous trees.
Or now. Two gaseous substances - oxygen and hydrogen, having connected together, turned into a liquid. Many other substances, including solid, connecting with hydrogen, become, as well as hydrogen, gaseous, for example, hydrogen sulfide H 2 S, selenium (H 2 SE), or a connection to Tellur (H 2 TE).
It is known that water is well dissolved by many substances. It is said that it dissolves, albeit into the disappearing small extent, even glass of glass, in which we poured it.
However, the most important thing to say about water is that water has become a lifestyle. Water, initially dissolving dozens of chemical compounds, that is, becoming sea water, has become unique in a variety of components of the component, which ultimately turned out to be a favorable environment for the origin and maintaining organic life.
In the first chapter of this our story, we have already noted that is almost generally recognized. The hypothesis now turned into the theory of the origin of life, each position of which, according to the authors of this theory, relies on the actual data of cosmogony, astronomy, historical geology, mineralogy, energy, physics, chemistry, including biological chemistry and other sciences.
The first is the opinion that life originated in the ocean, expressed the German natural scientist in 1893. He realized that the amazing similarity between blood and sea water in the composition of the salts dissolved in them is not random. Later, the theory of oceanic origin of the mineral composition of blood developed in detail the English physiologist McKellaum, who confirmed the correctness of this assumption by the results of numerous blood tests of various animals, starting with invertebrates and ending with mammals.
It turned out that not only blood, but the entire internal environment of our body demonstrates traces, preserved from the long stay of our distant ancestors in sea water.
Currently, world science has no doubt about the oceanic origin of life on Earth.
© Vladimir Kalanov,
"Knowledge is power"
11.07.2007 15:00
The world ocean is a single holistic natural body that takes 2/3 of the entire area of \u200b\u200bthe globe. Sea water from which it consists - the most common substance on the surface of the Earth. It differs from freshwater by bitter and salty taste, specific weight, transparency and color, more aggressive effect on building materials and other properties. This is due to the content of more than 50 different components in seawater.
The total content of solid solute substances in 1 kg of seawater and expressed in the decreases of percent (ppm) is called salinity. Middle salty Sea water on the surface of the ocean varies from 32 to 37, in natural layers from 34 to 35. In some seas there is a significant deviation from these averages. So, the salty of the Black Sea 17-18 ‰, the Caspian Sea 12-13, and the Red Sea - up to 40. Theoretically, all known chemical elements are in seawater, but their weight content is different.
Of the total amount of dissolved substances, 99.6% are halogen salts of sodium, potassium, magnesium and magnesium and calcium sulfates, and only 0.4% of the salt composition falls on the proportion of other substances. From the table, it can be seen that only 13 elements of the "Mendeleev Table" are contained in the amount of more than 0.1 mg / l. Even such important for many processes in the ocean (especially for the livelihoods of marine organisms) elements as phosphorus, iodine, iron, along with calcium, gray, carbon and some others are contained in an amount less than 0.1 mg / l. In seaside water in the form of live matter and in the form of dissolved "bone" organic substances, organic substances are also contained, amounting to a value of about 2 mg / l.
The salt composition of seawater differs sharply from the salt composition of river water, but close to the waters released at volcanic eruptions, or hot sources receiving food from the deep bowels of the earth. The river water also contains dissolved substances, the number of which is very dependent on physico-geographical conditions.
The greater the magnitude of evaporation, the greater the salinity of sea waterSince the evaporation remains salt. Oceanic and coastal flows, deposit of freshwater with large rivers, mixing the oceans and seas, have great influence on the change in salinity. In the depth of oscillation of salinity, only up to 1500 m occur, lower salinity changes slightly.
The most saline sea of \u200b\u200bthe World Ocean - Red. In 1 liter of its water contains 41 g of salts. On average, no more than 100 mm atmospheric precipitations fall over the sea over the sea, while evaporation from its surface reaches 2000 mm per year. With the absence of a river flow, it creates a constant shortage of the water balance of the sea, to replenish which there is only one source - the flow of water from the Gulf of Aden. During the year, through the Bab El Mongland, the strait in the sea is made about 1000 cubic meters. km of water more than being taken out of it. At the same time, according to the calculations, it takes only 15 years to complete the waters of the Red Sea.
In the Red Sea, the water is very good and uniformly mixed. In winter, the surface waters cooled, become more dense and lowered down, and up warm water from depth. In summer, water evaporates from the sea surface, and the remaining water becomes more salty, heavy and lowered down. Left water rises in her place. Thus, the whole year water in the sea is intensively mixed, and in all its volume the sea is the same in temperature and salinity, except in the depressions.
Detection wpadin with hot brine In the Red Sea, there was a real scientific discovery of the 60s of the twentieth century. To date, more than 20 such depressions have been discovered in the deepest areas. The brine temperature is within 30-60 ° C and rises by 0.3-0.7 ° C per year. This means that the depressions are heated below the inner heat of the Earth. Observers, immersed in the depressions on underwater devices, said that the brines do not merge with the surrounding water, and clearly differ from it and look like or soil, covered with ripples, or as a swinging fog. Chemical tests have shown that the content in brine of many metals, including precious, hundreds and thousands of times higher than in ordinary sea water.
The absence of coastal flow (and simply put the rivers and rainflows), which means and dirt from sushi, provides fabulous transparency of water.  The water temperature is stable all year round - 20-25 ° C. All these factors led to the wealth and uniqueness of marine life in the Red Sea.
The water temperature is stable all year round - 20-25 ° C. All these factors led to the wealth and uniqueness of marine life in the Red Sea.
Dead Sea Located in West Asia in Israel and Jordan. It is located in the tectonic wpadine, resulting from the so-called Afro-Asian fault, which occurred in the era somewhere between the end of the tertiary and the beginning of the Quaternary period, that is, more than 2 million years ago.
The area of \u200b\u200bthe Dead Sea is 1050 square meters. m, depth 350-400 meters. The only Jordan River flows into it, but the food occurs due to numerous mineral springs. The exit of the sea does not have, is unprecedented, therefore it is more correct to call him the lake.
The surface of the Dead Sea is located 400 meters below the level of the world's ocean (the lowest point of the globe). In his current outlines, the Dead Sea has existed more than 5,000 years, during which time a sedimental slim layer has a thickness of more than 100 meters in its day.
Instruction
The level of average salinity of the World Ocean is 35 ppm - it is this figure most often called statistics. A little more accurate value, without rounding: 34.73 ppm. In practice, this means that in each liter of the theoretical ocean water, about 35 g of salts should be dissolved. In practice, this value varies quite greatly, as the world ocean is so huge that the water in it cannot quickly mix and form a homogeneous space in terms of chemical properties.
The salinity of water in the ocean depends on several factors. First, it determines the percentage of water evaporated from the ocean and precipitation falling into it. If there are many precipitation, the level of local salinity falls, and if there is no precipitation, but the water is evaporated intensively, the salinity rises. Therefore, in the tropics in certain seasons, the salinity of water reaches a record values \u200b\u200bfor the planet. The most part of the ocean is the Red Sea, its salinity is 43 ppm.
At the same time, even if the content of salts on the surface of the sea or ocean fluctuates, usually these changes are practically not concerned with deep layers of water. Surface oscillations rarely exceed 6 ppm. In some areas, the salinity of water decreases due to the abundance of fresh rivers flowing into the sea.
The salinity of the quiet and altantic ocean is slightly higher than the others: it is 34.87 ppm. The Indian Ocean has a salinity of 34.58 ppm. The smallest salinity of the Arctic Ocean, and the reason for this is the melting of polar ice, which is particularly intensely happening in the southern hemisphere. The currents of the Arctic Ocean affect the Indian, which is why his salinity is lower than that of the Atlantic and Pacific Ocean.
The farther from the poles, the higher the salinity of the ocean, for the same reasons. However, the most saline latitudes are from 3 to 20 degrees in both directions from the equator, and he does not at all. Sometimes these "strips" even say that this is a belt salinity. The reason for such a distribution is that the equator is the area of \u200b\u200bconstant loss of abundant pouring tropical rains that are cleaned with water.
Video on the topic
note
Not only salinity changes, but also the water temperature in the World Ocean. Horizontally temperature changes from the equator to the poles, but the vertical change in temperature also takes place: it decreases to the depth. The reason is that the sun is not able to penetrate through the entire watertime and heat the water of the ocean until the bottom. The temperature of the water surface varies very much. In the area of \u200b\u200bthe equator, it reaches + 25-28 degrees Celsius, and not far from the North Pole can be lowered to 0, and sometimes it happens slightly lower.
Helpful advice
The Ocean Square is about 360 million square meters. km. It is about 71% of the entire territory of the planet.