Where High Density Tungsten Is Applied. Density of tungsten
Read also
Tungsten also belongs to the group of metals with high refractories. It was discovered in Sweden by a chemist named Scheele. It was he who was the first to isolate an oxide of an unknown metal from the wolframite mineral in 1781. The scientist managed to obtain tungsten in its pure form after 3 years.
Description
Tungsten belongs to a group of materials that are often used in various industries. He denoted by the letter W and in the periodic table has a serial number 74. It is characterized by a light gray color. One of its characteristic qualities is high refractoriness. The melting point of tungsten is 3380 degrees Celsius. If we consider it from the point of view of application, then the most important qualities of this material are:
- density;
- melting temperature;
- electrical resistance;
- coefficient of linear expansion.
When calculating its characteristic qualities, it is necessary to highlight the high boiling point, which is located on at the level of 5 900 degrees Celsius... Another feature is its low evaporation rate. It is not high even in temperature conditions of 2000 degrees Celsius. For such a property as electrical conductivity, this metal is 3 times higher than such a common alloy as copper.
Factors limiting the use of tungsten
There are a number of factors that limit the use of this material:
- high density;
- significant tendency to brittleness at low temperatures;
- low resistance to oxidation.
By its appearance, tungsten resembles conventional steel... Its main application is mainly associated with the production of alloys with high strength characteristics. This metal can be processed, but only if it is preheated. Depending on the type of treatment chosen, heating is performed to a certain temperature. For example, if the task is to forge rods from tungsten, then the workpiece must be preheated to a temperature of 1450-1500 degrees Celsius.
For 100 years, tungsten has not been used commercially. Its use in the production of various techniques was restrained by its high melting point.
The beginning of its industrial use is associated with 1856, when it was first used for alloying tool steel grades. During their production, tungsten was added to the composition with a total share of up to 5%. The presence of this metal in the composition of steel made it possible to increase the cutting speed on lathes from 5 to 8 m per minute.
The development of industry in the second half of the 19th century is characterized by the active development of the machine tool industry. The demand for equipment was constantly growing every year, which required machine builders to obtain high-quality characteristics of machines, and in addition to this, increase their operating speed. The first impetus for increasing cutting speed was the use of tungsten.
Already at the beginning of the 20th century, the cutting speed was increased up to 35 meters per minute... This was achieved by alloying steel not only with tungsten, but also with other elements:
- molybdenum;
- chrome;
- vanadium.
Subsequently, the cutting speed on the machines increased to 60 meters per minute. But, despite such high rates, experts understood that there was an opportunity to improve this characteristic. The specialists did not think for a long time what method to choose to increase the cutting speed. They resorted to using tungsten, but already in the form of carbides in alliance with other metals and their types. Nowadays, it is quite common to cut metal on machines of 2000 meters per minute.
 Like any material, tungsten has its own special properties, thanks to which it fell into the group of strategic metals. We have already said above that one of the advantages of this metal is its high refractoriness. It is due to this property that the material can be used for the manufacture of filaments.
Like any material, tungsten has its own special properties, thanks to which it fell into the group of strategic metals. We have already said above that one of the advantages of this metal is its high refractoriness. It is due to this property that the material can be used for the manufacture of filaments.
Its melting point is at 2500 degrees Celsius... But only this quality is not limited to the positive properties of this material. It also has other advantages that should be mentioned. One of them is the high strength demonstrated under normal and high temperature conditions. For example, when iron and alloys based on it are heated to a temperature of 800 degrees Celsius, the strength decreases by 20 times. Under the same conditions, the strength of tungsten decreases only three times. In conditions of 1500 degrees Celsius, the strength of iron is practically reduced to zero, but in tungsten it is at the level of iron at ordinary temperatures.
Today 80% of the tungsten produced in the world is mainly used in the manufacture of high quality steel. More than half of the steel grades used by machine-building enterprises contain tungsten. They use them as their main material. for turbine parts, gearboxes, and also use such materials for the manufacture of compressor machines. Tungsten-containing engineering steels are used to produce shafts, gears, and a one-piece forged rotor.
In addition, they are used for the manufacture of crankshafts, connecting rods. The addition of tungsten and other alloying elements to the composition of machine-building steel increases their hardenability. In addition, it is possible to obtain a fine-grained structure. Along with this, characteristics such as hardness and strength of the produced machine-building steels increase.
In the production of heat-resistant alloys, the use of tungsten is one of the prerequisites. The need to use this particular metal is due to the fact that it is the only one that is able to withstand significant loads at high temperatures exceeding the value of iron melting. Tungsten and compounds based on this metal are characterized by high strength and good elasticity. In this regard, they are superior to other metals included in the group of refractory materials.
Minuses
However, listing the advantages of tungsten, it should be noted and disadvantages that are inherent in this material.

Tungsten, which is currently produced, contains 2% thorium. This alloy is called thoriated tungsten. It is characterized by tensile strength 70 MPa at a temperature of 2420 degrees Celsius. Although the value of this indicator is not high, we note that only 5 metals, together with tungsten, do not change their solid state at such a temperature.
This group includes molybdenum, which has a melting point of 2625 degrees. Another metal is technetium. However, alloys based on it are unlikely to be produced in the near future. Rhenium and tantalum are not highly durable under these temperature conditions. Therefore, tungsten is the only material that is able to provide sufficient strength under high temperature loads. For the reason that it is among the scarce ones, if there is an opportunity to replace it, then manufacturers use an alternative to it.
However, in the production of individual components, there are no materials that could fully replace tungsten. For example, in the manufacture of filaments of electric lamps and anodes of DC arc lamps, only tungsten is used, since there are simply no suitable substitutes. Also it is used in the manufacture of electrodes for argon-arc and atomic-hydrogen welding. Also, using this material, a heating element is manufactured that is used in conditions from 2000 degrees Celsius.
Application
 Tungsten and alloys based on it are widely used in various industries. They are used in the production of aircraft engines, used in the field of rocketry, as well as for the production of space technology. In these areas, using these alloys, jet nozzles and inserts of critical sections in rocket engines are made. In addition, such materials are used as basic materials for the manufacture of rockets alloys.
Tungsten and alloys based on it are widely used in various industries. They are used in the production of aircraft engines, used in the field of rocketry, as well as for the production of space technology. In these areas, using these alloys, jet nozzles and inserts of critical sections in rocket engines are made. In addition, such materials are used as basic materials for the manufacture of rockets alloys.
The production of alloys from this metal has one feature that is associated with the refractoriness of this material. At high temperatures, many metals change their state and turn into gases or highly volatile liquids. Therefore, to obtain alloys in which tungsten is present, methods of powder metallurgy are used.
Such methods involve pressing a mixture of metal powders, subsequent sintering and further subjecting them to arc melting, carried out in electrode furnaces. In some cases, the sintered tungsten powder is additionally impregnated with a liquid solution of some other metal. Thus, pseudo-alloys of tungsten, copper, silver are obtained, which are used for contacts in electrical installations. Compared with copper, the durability of such products is 6-8 times higher.
This metal and its alloys have great prospects for further expanding the scope of application. First of all, it should be noted that, in contrast to nickel, these materials can work at the "fiery" frontiers. The use of tungsten products instead of nickel leads to the increased operating parameters of power plants. And this leads to increasing equipment efficiency... In addition, tungsten-based products can withstand harsh environments with ease. Thus, we can confidently state that tungsten will continue to lead the group of such materials in the near future.
 Tungsten also contributed to the process of improving the electric incandescent lamp. Until the period of 1898, carbon filament was used in these electric lighting fixtures.
Tungsten also contributed to the process of improving the electric incandescent lamp. Until the period of 1898, carbon filament was used in these electric lighting fixtures.
- it was easy to manufacture;
- its production was inexpensive.
The only drawback of the charcoal filament was that life time she had a small one. After 1898, the carbon filament of lamps had a competitor in the form of osmium. Beginning in 1903, tantalum was used for the production of electric lamps. However, already in 1906, tungsten replaced these materials and began to be used for the manufacture of filaments for incandescent lamps. It is also used today in the manufacture of modern light bulbs.
To provide this material with high heat resistance, a layer of rhenium and thorium is applied to the metal surface. In some cases, the tungsten filament is made with the addition of rhenium. This is due to the fact that at high temperatures this metal begins to evaporate, and this leads to the fact that the thread of this material becomes thinner. The addition of rhenium to the composition leads to a 5-fold decrease in the effect of evaporation.
Nowadays, tungsten is actively used not only in the production of electrical engineering, but also various military-industrial products... Its addition to weapon steel provides high performance for this type of material. In addition, it allows you to improve the characteristics of armor protection, as well as to make armor-piercing shells more effective.
Conclusion
 Tungsten is one of the demanded materials used in metallurgy. Adding it to the composition of the produced steels provides an increase in their characteristics. They become more resistant to thermal stress, and in addition, the melting temperature rises, which is especially important for products used in extreme conditions. at high temperatures... The use in the manufacture of various equipment, products and elements, assemblies made of this metal or alloys based on it, allows you to improve the characteristics of equipment and increase the efficiency of their work.
Tungsten is one of the demanded materials used in metallurgy. Adding it to the composition of the produced steels provides an increase in their characteristics. They become more resistant to thermal stress, and in addition, the melting temperature rises, which is especially important for products used in extreme conditions. at high temperatures... The use in the manufacture of various equipment, products and elements, assemblies made of this metal or alloys based on it, allows you to improve the characteristics of equipment and increase the efficiency of their work.
Tungsten- the most refractory metal. Only the non-metallic element, carbon, has a higher melting point. Chemically resistant under standard conditions. The name Wolframium was transferred to the element from the mineral wolframite, known as far back as the 16th century. called lat. Spuma lupi ("wolf foam") or him. Wolf Rahm ("wolf cream", "wolf cream"). The name was due to the fact that tungsten, accompanying tin ores, interfered with the smelting of tin, transforming it into a foam of slags ("devours tin like a wolf a sheep").
See also:
STRUCTURE
 A tungsten crystal has a body-centered cubic lattice. Tungsten crystals in the cold are characterized by low plasticity, therefore, in the process of pressing the powder, they practically do not practically change their basic shape and size, and the compaction of the powder occurs mainly through the relative movement of particles.
A tungsten crystal has a body-centered cubic lattice. Tungsten crystals in the cold are characterized by low plasticity, therefore, in the process of pressing the powder, they practically do not practically change their basic shape and size, and the compaction of the powder occurs mainly through the relative movement of particles. In a body-centered cubic tungsten cell, atoms are located at the vertices and in the center of the cell, i.e. there are two atoms per cell. The bcc structure is not the closest packing of atoms. The compactness factor is 0.68. Space group of tungsten Im3m.
PROPERTIES
 Tungsten is a shiny light gray metal with the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seborgium is very short). Melting point - 3695 K (3422 ° C), boils at 5828 K (5555 ° C). The density of pure tungsten is 19.25 g / cm³. Possesses paramagnetic properties (magnetic susceptibility 0.32 · 10-9). Brinell hardness 488 kg / mm², electrical resistivity at 20 ° C - 55 · 10−9 Ohm · m, at 2700 ° C - 904 · 10−9 Ohm · m. The speed of sound in annealed tungsten is 4290 m / s. It is a paramagnetic.
Tungsten is a shiny light gray metal with the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seborgium is very short). Melting point - 3695 K (3422 ° C), boils at 5828 K (5555 ° C). The density of pure tungsten is 19.25 g / cm³. Possesses paramagnetic properties (magnetic susceptibility 0.32 · 10-9). Brinell hardness 488 kg / mm², electrical resistivity at 20 ° C - 55 · 10−9 Ohm · m, at 2700 ° C - 904 · 10−9 Ohm · m. The speed of sound in annealed tungsten is 4290 m / s. It is a paramagnetic.
Tungsten is one of the heaviest, hardest and most refractory metals. In its pure form, it is a silver-white metal, similar to platinum, at a temperature of about 1600 ° C it lends itself well to forging and can be drawn into a thin thread.
RESERVES AND PRODUCTION
 The clarke of tungsten in the earth's crust is (according to Vinogradov) 1.3 g / t (0.00013% by content in the earth's crust). Its average content in rocks, ppm: ultrabasic - 0.1, basic - 0.7, medium - 1.2, acidic - 1.9.
The clarke of tungsten in the earth's crust is (according to Vinogradov) 1.3 g / t (0.00013% by content in the earth's crust). Its average content in rocks, ppm: ultrabasic - 0.1, basic - 0.7, medium - 1.2, acidic - 1.9.
The process of obtaining tungsten passes through a substage of separation of WO 3 trioxide from ore concentrates and subsequent reduction to metal powder with hydrogen at a temperature of about 700 ° C. Due to the high melting point of tungsten, powder metallurgy methods are used to obtain a compact form: the resulting powder is pressed, sintered in a hydrogen atmosphere at a temperature of 1200-1300 ° C, then an electric current is passed through it. The metal is heated to 3000 ° C, while sintering into a monolithic material occurs. Zone melting is used for subsequent purification and obtaining a monocrystalline form.
ORIGIN
 Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO 3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Of industrial importance are wolframite (iron and manganese tungstate nFeWO 4 * mMnWO 4 - ferberite and hubnerite, respectively) and scheelite (calcium tungstate CaWO 4). Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO 3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Of industrial importance are wolframite (iron and manganese tungstate nFeWO 4 * mMnWO 4 - ferberite and hubnerite, respectively) and scheelite (calcium tungstate CaWO 4). Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
The largest reserves are in Kazakhstan, China, Canada and the United States; deposits in Bolivia, Portugal, Russia, Uzbekistan and South Korea are also known. World production of tungsten is 49-50 thousand tons per year, including 41 in China, 3.5 in Russia; Kazakhstan 0.7, Austria 0.5. The main exporters of tungsten: China, South Korea, Austria. Main importers: USA, Japan, Germany, Great Britain.
There are also tungsten deposits in Armenia and other countries.
APPLICATION
 The refractoriness and plasticity of tungsten make it indispensable for filaments in lighting fixtures, as well as in picture tubes and other vacuum tubes.
The refractoriness and plasticity of tungsten make it indispensable for filaments in lighting fixtures, as well as in picture tubes and other vacuum tubes.
Due to its high density, tungsten is the basis of heavy alloys, which are used for counterweights, armor-piercing cores of sub-caliber and arrow-shaped feathered shells of artillery guns, cores of armor-piercing bullets and ultra-high-speed gyro rotors to stabilize the flight of ballistic missiles (up to 180 thousand rpm).
Tungsten is used as electrodes for argon arc welding. Alloys containing tungsten are characterized by high temperature resistance, acid resistance, hardness and abrasion resistance. They are used to make surgical instruments (amaloy alloy), tank armor, torpedo and shell shells, the most important parts of aircraft and engines, containers for storing radioactive substances. Tungsten is an important component of the best grades of tool steels. Tungsten is used in high-temperature vacuum resistance furnaces as heating elements. An alloy of tungsten and rhenium is used in such furnaces as a thermocouple.
For mechanical processing of metals and non-metallic structural materials in mechanical engineering (turning, milling, planing, chiseling), drilling, in the mining industry, hard alloys and composite materials based on tungsten carbide are widely used (for example, win, consisting of WC crystals in a cobalt matrix; grades widely used in Russia - VK2, VK4, VK6, VK8, VK15, VK25, T5K10, T15K6, T30K4), as well as mixtures of tungsten carbide, titanium carbide, tantalum carbide (TT grades for particularly severe processing conditions, for example, chiselling and planing forgings of heat-resistant steels and rotary hammer drilling of hard material). It is widely used as an alloying element (often in conjunction with molybdenum) in iron-based steels and alloys. High-alloy steels of the "high speed" class, with markings starting with the letter P, almost always contain tungsten. (P18, P6M5. From rapid - fast, speed).
Tungsten sulfide WS 2 is used as a high-temperature (up to 500 ° C) grease. Some tungsten compounds are used as catalysts and pigments. Monocrystals of tungstates (tungstates of lead, cadmium, calcium) are used as scintillation detectors of X-rays and other ionizing radiation in nuclear physics and nuclear medicine.
Tungsten ditelluride WTe 2 is used to convert thermal energy into electrical energy (thermo-EMF about 57 μV / K). Artificial radionuclide 185 W is used as a radioactive label in substance research. Stable 184 W is used as a component of alloys with uranium-235 used in solid-state nuclear rocket engines, since it is the only common tungsten isotope with a low thermal neutron capture cross section (about 2 barn).
Tungsten - W
CLASSIFICATION
| Nickel-Strunz (10th Edition) | 1.AE.05 |
| Dana (7th Edition) | 1.1.38.1 |
Tungsten stands out among metals not only by its refractoriness, but also by its mass. The density of tungsten under normal conditions is 19.25 g / cm³, which is about 6 times that of aluminum. Compared to copper, tungsten is 2 times heavier. At first glance, high density may seem like a disadvantage, because products made from it will be heavy. But even this feature of the metal has found its application in technology. Benefits of tungsten due to its high density:
- The ability to concentrate a large mass in a small volume.
- Protection against ionizing radiation (radiation).
The first property is explained by the internal structure of the metal. The nucleus of an atom contains 74 protons and 110 neutrons, that is, 184 particles. In the Periodic Table of Chemical Elements, in which atoms are arranged in ascending order of atomic mass, tungsten is in 74th place. For this reason, a substance composed of heavy atoms will have a large mass. Radiation shielding is inherent in all high density materials. This is due to the fact that ionizing radiation, colliding with any obstacle, transfers it part of its energy. Denser substances have a high concentration of particles per unit volume, therefore, ionizing rays undergo more collisions and, accordingly, lose more energy. The use of the metal is based on the above properties.
Application of tungsten
High density is a huge advantage of tungsten over other metals.
Tungsten is widely used in various industries.
Uses based on a large mass of metal
Its considerable density makes tungsten a valuable balancing material. Balancing weights made from it reduce the load acting on the parts. Thus, their operational period is extended. Tungsten Applications:
- Aerospace. Heavy metal parts balance the effective torques. Therefore, tungsten is used for the manufacture of helicopter blades, propellers, rudders. Due to the fact that the material does not possess magnetic properties, it is used in the production of on-board electronic systems of aviation.
- Automotive industry. Tungsten is used where it is necessary to concentrate a large mass in a small volume of space, for example, in automobile engines installed on heavy trucks, expensive SUVs, and diesel vehicles. Also, tungsten is an advantageous material for the manufacture of crankshafts and flywheels, chassis loads. In addition to its high density, the metal is characterized by a high modulus of elasticity, thanks to these qualities it is used to damp vibrations on drives.
- Optics. Tungsten weights of complex configuration act as balancers in microscopes and other high-precision optical instruments.
- Sports equipment production. Tungsten is used instead of lead in sports equipment because, unlike the latter, it is not harmful to health and the environment. For example, the material is used in the manufacture of golf clubs.
- In mechanical engineering. Vibrating hammers are made of tungsten, with which they drive piles. There is a rotating weight in the middle of each instrument. It converts vibration energy into driving force. Due to the presence of tungsten, it is possible to use vibratory hammers for compacted soil of considerable thickness.
- For the manufacture of precision instruments. In deep drilling, precision instruments are used, the holder of which must not be subject to vibration. This requirement is met by tungsten, which also has a high modulus of elasticity. Anti-vibration holders provide smooth operation, so they are used in boring and grinding bars, in tool shafts. The working part of the tool is made on the basis of tungsten, since it has increased hardness.
Use based on the ability to protect against radiation

Tungsten collimators in surgery.
- According to this criterion, tungsten alloys are ahead of cast iron, steel, lead and water, therefore collimators and protective screens are made of metal, which are used in radiotherapy. Tungsten alloys are not subject to deformation and are highly reliable. The use of multi-leaf collimators makes it possible to direct radiation to a specific area of the affected tissue. During therapy, X-rays are taken first to localize the location and determine the nature of the tumor. Then the collimator petals are moved by the electric motor to the desired position. 120 petals can be used, with the help of which a field is created that repeats the shape of the tumor. Further, beams with high radiation are directed to the affected area. In this case, the tumor is irradiated by the fact that the multi-leaf collimator rotates around the patient. To protect adjacent healthy tissues and the environment from radiation, the collimator must be highly accurate.
- Special ring collimators made of tungsten for radiosurgery have been developed, the irradiation of which is directed to the head and neck. The device carries out high-precision focusing of gamma radiation. Also, tungsten is a part of plates for computer tomographs, shielding elements for detectors and linear accelerators, dosimetry equipment and non-destructive testing devices, containers for radioactive substances. Tungsten is used in drilling devices. Shields are made of it to protect immersed instruments from X-ray and gamma radiation.
Tungsten alloy classification
Criteria such as the increased density and refractoriness of tungsten make it possible to use it in many industries. However, modern technologies sometimes require additional material properties that pure metal does not have. For example, its electrical conductivity is lower than that of copper, and the manufacture of a part of a complex geometric shape is difficult due to the fragility of the material. In such situations, impurities help. Moreover, their number often does not exceed 10%. After adding copper, iron, nickel, tungsten, the density of which remains very high (not less than 16.5 g / cm³), conducts electric current better and becomes ductile, which makes it possible to process it well.

Residence permit, VNM, VD
Depending on the composition, alloys are marked differently.
- VNZH are tungsten alloys that contain nickel and iron,
- ВНМ - nickel and copper,
- VD - only copper.
In the labeling, capital letters are followed by numbers indicating the percentage. For example, VNM 3–2 is a tungsten alloy with the addition of 3% nickel and 2% copper, VNM 5–3 contains 5% nickel and 3% iron in the impurity, VD-30 consists of 30% copper.
Tungsten belongs to refractory metals, which are relatively rare in the earth's crust. Thus, the content in the earth's crust (in%) of tungsten is about 10 -5, rhenium 10 -7, molybdenum 3.10 -4, niobium 10 -3, tantalum 2.10 -4 and vanadium 1.5.10 -2.
Refractory metals are transitional elements and are located in groups IV, V, VI and VII (subgroup A) of the periodic table of elements. With an increase in the atomic number, the melting point of refractory metals in each of the subgroups increases.
Elements VA and VIA groups (vanadium, niobium, tantalum, chromium, molybdenum and tungsten) are refractory metals with a body-centered cubic lattice, unlike other refractory metals, which have a face-centered and hexagonal densely packed structure.
It is known that the main factor determining the crystal structure and physical properties of metals and alloys is the nature of their interatomic bonds. Refractory metals are characterized by a high strength of the interatomic bond and, as a consequence, a high melting point, increased mechanical strength, and significant electrical resistance.
The possibility of studying metals by electron microscopy makes it possible to study the structural features of the atomic scale, reveals the relationship between mechanical properties and dislocations, stacking faults, etc. The data obtained show that the characteristic physical properties that distinguish refractory metals from ordinary ones are determined by the electronic structure of their atoms. Electrons can pass to varying degrees from one atom to another, and the type of transition corresponds to a certain type of interatomic bond. The peculiarity of the electronic structure determines the high level of interatomic forces (bonds), high melting point, strength of metals and their interaction with other elements and impurities of introduction. In tungsten, the energetically active shell includes 5 d and 6 s electrons.
Of the refractory metals, tungsten has the highest density - 19.3 g / cm 3. Although, when used in structures, the high density of tungsten can be considered as a negative indicator, nevertheless, the increased strength at high temperatures makes it possible to reduce the mass of tungsten products by reducing their size.
The density of refractory metals depends to a large extent on their condition. For example, the density of a sintered tungsten rod ranges from 17.0-18.0 g / cm 3, and the density of a forged rod with a deformation rate of 75% is 18.6-19.2 g / cm 3. The same is observed in molybdenum: a sintered bar has a density of 9.2-9.8 g / cm 3, forged with a deformation degree of 75% -9.7-10.2 g / cm 3 and cast 10.2 g / cm 3 ...
Some physical properties of tungsten, tantalum, molybdenum and niobium for comparison are given in table. 1. The thermal conductivity of tungsten is less than half the thermal conductivity of copper, but it is much higher than that of iron or nickel.
Refractory metals of groups VA, VIA, VIIA of the periodic table of elements have a lower coefficient of linear expansion in comparison with other elements. Tungsten has the lowest coefficient of linear expansion, which indicates the high stability of its atomic lattice and is a unique property of this metal.
Tungsten has a thermal conductivity about 3 times less than the electrical conductivity of annealed copper, but it is higher than that of iron, platinum and phosphorite bronze.
For metallurgy, the density of the metal in the liquid state is of great importance, since this characteristic determines the speed of movement along the channels, the process of removing gaseous and non-metallic inclusions and affects the formation of a shrinkage cavity and porosity in ingots. For tungsten, this value is higher than for other refractory metals. However, another physical characteristic — the surface tension of liquid refractory metals at the melting temperature — differs less (see Table 1). Knowledge of this physical property is essential in processes such as protective coating, impregnation, melting and casting.
An important casting property of a metal is fluidity. If for all metals this value is determined when pouring a liquid metal into a spiral form at a pouring temperature higher than the melting temperature by 100-200 ° C, then the fluidity of tungsten is obtained by extrapolating the empirical dependence of this value on the heat of fusion.
Tungsten is stable in various gases, acids and some molten metals. At room temperature, tungsten does not interact with hydrochloric, sulfuric and phosphoric acids, is not exposed to dissolved nitric acid and, to a lesser extent than molybdenum, reacts to a mixture of nitric and hydrofluoric acids. Tungsten has high corrosion resistance in the environment of some alkalis, for example, in the environment of sodium and potassium hydroxide, in which it is resistant to temperatures of 550 ° C. When exposed to molten sodium, it is stable up to 900 ° C, mercury up to 600 ° C, gallium up to 800 and bismuth up to 980 ° C. The corrosion rate in these liquid metals does not exceed 0.025 mm / year. At a temperature of 400-490 ° C, tungsten begins to oxidize in air and oxygen. A weak reaction occurs when heated to 100 ° C in hydrochloric, nitric and hydrofluoric acids. In a mixture of hydrofluoric and nitric acids, tungsten dissolves rapidly. Interaction with gaseous media begins at temperatures (° C): with chlorine 250, with fluorine 20. In carbon dioxide, tungsten is oxidized at 1200 ° C, in ammonia the reaction does not occur.
The regularity of the oxidation of refractory metals is mainly determined by the temperature. Tungsten up to 800-1000 ° C has a parabolic oxidation pattern, and above 1000 ° C it is linear.
High corrosion resistance in liquid metal media (sodium, potassium, lithium, mercury) allows the use of tungsten and its alloys in power plants.
The strength properties of tungsten depend on the state of the material and temperature. For forged tungsten rods, the tensile strength after recrystallization varies depending on the test temperature from 141 kgf / mm 2 at 20 ° C to 15.5 kgf / mm 2 at 1370 ° C. Tungsten obtained by powder metallurgy when the temperature changes from 1370 to 2205 ° C has? b = 22.5 × 6.3 kgf / mm 2. The strength of tungsten increases especially during cold deformation. A wire with a diameter of 0.025 mm has a tensile strength of 427 kgf / mm 2.
The hardness of deformed commercially pure tungsten HB 488, annealed HB 286. At the same time, such a high hardness remains up to temperatures close to the melting point, and largely depends on the purity of the metal.
The elastic modulus is approximately related to the atomic volume of the melting temperature

where T pl is the absolute melting point; V aТ - atomic volume; K is a constant.
A distinctive feature of tungsten among metals is also a high volumetric deformation, which is determined from the expression

where E is the modulus of elasticity of the first kind, kgf / mm 2; ? -coefficient of transverse deformation.
Tab. 3 illustrates the change in volumetric deformation for steel, cast iron and tungsten, calculated using the above expression.

The plasticity of commercially pure tungsten at 20 ° C is less than 1% and increases after electron-beam zone cleaning from impurities, as well as when doped with the addition of 2% thorium oxide. With increasing temperature, the plasticity increases.
 The high energy of interatomic bonds of metals of groups IV, V, VIA determines their high strength at room and elevated temperatures. The mechanical properties of refractory metals substantially depend on their purity, production methods, mechanical and thermal treatment, the type of semi-finished products and other factors. Most of the information on the mechanical properties of refractory metals, published in the literature, was obtained on insufficiently pure metals, since melting under vacuum conditions began to be used relatively recently.
The high energy of interatomic bonds of metals of groups IV, V, VIA determines their high strength at room and elevated temperatures. The mechanical properties of refractory metals substantially depend on their purity, production methods, mechanical and thermal treatment, the type of semi-finished products and other factors. Most of the information on the mechanical properties of refractory metals, published in the literature, was obtained on insufficiently pure metals, since melting under vacuum conditions began to be used relatively recently.
In fig. 1 shows the dependence of the melting point of refractory metals on the position in the periodic table of elements.
Comparison of the mechanical properties of tungsten after arc melting and tungsten obtained by the method of powder metallurgy shows that although their ultimate strength differs insignificantly, arc melting tungsten turns out to be more ductile.
The Brinell hardness of tungsten in the form of a sintered bar is HB 200-250, and of rolled cold-worked sheet HB 450-500, the hardness of molybdenum is HB 150-160 and HB 240-250, respectively.
Alloying of tungsten is carried out in order to increase its plasticity, for this purpose, substitutional elements are used. More and more attention is paid to attempts to increase the ductility of metals of group VIA by adding small amounts of elements of groups VII and VIII. The increase in plasticity is explained by the fact that when alloying transition metals with additives, an inhomogeneous electron density is created in the alloy due to the localization of electrons of the alloying elements. In this case, the atom of the alloying element changes the forces of interatomic bonds in the adjacent volume of the solvent; the length of such a volume should depend on the electronic structure of the alloyed and alloyed metals.
The difficulty in creating tungsten alloys is that it has not yet been possible to provide the necessary ductility with increasing strength. The mechanical properties of tungsten alloys doped with molybdenum, tantalum, niobium and thorium oxide (during short-term tests) are given in table. 4.

Alloying tungsten with molybdenum makes it possible to obtain alloys, which in their strength properties are superior to unalloyed tungsten up to temperatures of 2200 ° C (see Table 4). With an increase in the tantalum content from 1.6 to 3.6% at a temperature of 1650 ° C, the strength increases 2.5 times. This is accompanied by a 2-fold decrease in elongation.
Precipitation-hardened and complex-alloyed tungsten-based alloys, which contain molybdenum, niobium, hafnium, zirconium, and carbon, have been developed and are being mastered. For example, the following compositions: W - 3% Mo - 1% Nb; W - 3% Mo - 0.1% Hf; W - 3% Mo - 0.05% Zr; W - 0.07% Zr - 0.004% B; W - 25% Mo - 0.11% Zr - 0.05% C.
Alloy W - 0.48% Zr-0.048% C has? b = 55.2 kgf / mm 2 at 1650 ° C and 43.8 kgf / mm 2 at 1925 ° C.
Tungsten alloys containing thousandths of a percent of boron, tenths of a percent of zirconium, and hafnium and about 1.5% of niobium have high mechanical properties. The tensile strength of these alloys at high temperatures is 54.6 kgf / mm 2 at 1650 ° C, 23.8 kgf / mm 2 at 2200 ° C and 4.6 kgf / mm 2 at 2760 ° C. However, the transition temperature (about 500 ° C) of such alloys from the plastic state to the brittle state is quite high.
The literature contains information on tungsten alloys with 0.01 and 0.1% C, which are characterized by a tensile strength that is 2-3 times higher than the tensile strength of recrystallized tungsten.
Rhenium significantly increases the heat resistance of tungsten alloys (Table 5).

For a very long time and on a large scale, tungsten and its alloys have been used in electrical and vacuum technology. Tungsten and its alloys are the main material for the manufacture of filaments, electrodes, cathodes and other structural elements of powerful electric vacuum devices. High emissivity and light output in an incandescent state, low vapor pressure make tungsten one of the most important materials for this industry. Pure (no additives) tungsten is used in electrovacuum devices for the manufacture of parts operating at low temperatures that are not pretreated at temperatures above 300 ° C.
Additives of various elements significantly change the properties of tungsten. This makes it possible to create tungsten alloys with the required characteristics. For example, for parts of electrovacuum devices that require the use of non-sagging tungsten at temperatures up to 2900 ° C and with a high temperature of primary recrystallization, alloys with silicon-alkali or aluminum additives are used. Silicon alkali and thorium additives increase the recrystallization temperature and increase the strength of tungsten at high temperatures, which makes it possible to manufacture parts operating at temperatures up to 2100 ° C under conditions of increased mechanical stress.
Cathodes of electronic and gas-discharge devices, hooks and springs of generator lamps in order to increase the emission properties are made of tungsten with an additive of thorium oxide (for example, grades VT-7, VT-10, VT-15, with the content of thorium oxide, respectively, 7, 10 and 15% ).
High-temperature thermocouples are made from tungsten-rhenium alloys. Tungsten without additives, in which a high content of impurities is allowed, is used in the manufacture of cold parts of electrovacuum devices (glass bushings, crossheads). It is recommended to make the electrodes of flash lamps and cold cathodes of gas-discharge lamps from an alloy of tungsten with nickel and barium.
For operation at temperatures above 1700 ° C, BB-2 alloys (tungsten-moniobium) should be used. It is interesting to note that in short-term tests, alloys with niobium content from 0.5 to 2% have a tensile strength at 1650 ° C 2-2.5 times higher than unalloyed tungsten. The most durable alloy is tungsten with 15% molybdenum. W-Re-Th O 2 alloys have good machinability compared to W-Re alloys; the addition of thorium dioxide makes processing such as turning, milling, drilling possible.
Alloying tungsten with rhenium increases its plasticity, while the strength properties become approximately the same with increasing temperature. The addition of fine oxides to tungsten alloys increases their ductility. In addition, these additives significantly improve the machinability.
Alloys of tungsten with rhenium (W - 3% Re; W - 5% Re; W - 25% Re) are used to measure and control temperatures up to 2480 ° C in steel production and in other types of technology. The use of tungsten-rhenium alloys in the manufacture of anti-cathodes in X-ray tubes is increasing. Molybdenum anti-cathodes coated with this alloy work under high stress and have a longer service life.
The high sensitivity of tungsten electrodes to changes in the concentration of hydrogen ions allows them to be used for potentiometric titration. Such electrodes are used to control water and various solutions. They are simple in design and have a low electrical resistance, which makes them promising as microelectrodes in the study of acid resistance of the near-electrode layer in electrochemical processes.
The disadvantages of tungsten are its low ductility (?<1%), большая плотность, высокое поперечное сечение захвата тепловых нейтронов, плохая свариваемость, низкая ока-линостойкость и плохая обрабатываемость резанием. Однако легирование его различными элементами позволяет улучшить эти характеристики.
A number of parts for the electrical industry and engine nozzle liners are made of tungsten impregnated with copper or silver. The interaction of the refractory solid phase (tungsten) with the impregnating metal (copper or silver) is such that the mutual solubility of the metals is practically absent. The contact angles of tungsten wetting with liquid copper and silver are rather small due to the high surface energy of tungsten, and this fact improves the penetration of silver or copper. Tungsten impregnated with silver or copper was produced initially by two methods: by completely immersing a tungsten billet in molten metal or by partially immersing a suspended tungsten billet. There are also methods of impregnation using liquid hydrostatic pressure or vacuum suction.
The manufacture of electrical contacts impregnated with silver or copper from tungsten is carried out as follows. First, the tungsten powder is pressed and sintered under certain technological conditions. Then the resulting workpiece is impregnated. Depending on the obtained porosity of the workpiece, the proportion of the impregnating agent changes. Thus, the copper content in tungsten can vary from 30 to 13% when the specific pressing pressure changes from 2 to 20 tf / cm 2. The technology for obtaining impregnated materials is quite simple, economical, and the quality of such contacts is higher, since one of the components gives the material high hardness, erosion resistance, a high melting point, and the other increases its electrical conductivity.
Good results are obtained when using impregnated tungsten with copper or silver for the manufacture of nozzle inserts for solid fuel engines. An increase in such properties of impregnated tungsten as thermal conductivity and electrical conductivity, coefficient of thermal expansion, significantly increases the durability of the engine. In addition, the evaporation of the impregnating metal from the tungsten during engine operation has a positive effect, reducing heat fluxes and reducing the erosive effect of combustion products.
Tungsten powder is used in the manufacture of porous materials for parts of an electrostatic ion engine. The use of tungsten for these purposes makes it possible to improve its basic characteristics.
Thermal erosion properties of nozzles made of tungsten reinforced with dispersed oxides ZrO2, MgO2, V2O3, НfO2 increase in comparison with sintered tungsten nozzles. After appropriate preparation, galvanic coatings are applied to the tungsten surface to reduce high-temperature corrosion, for example, nickel plating, which is performed in an electrolyte containing 300 g / l of sodium sulfate, 37.5 g / l of boric acid at a current density of 0.5-11 A / dm 2, temperature 65 ° С and pH = 4.
Tungsten is a Group 4 chemical element with atomic number 74 in the periodic table of Dmitry Ivanovich Mendeleev, denoted W (Wolframium). The metal was discovered and isolated by two Spanish chemical scientists, the brothers d'Eluyard, in 1783. The very name "Wolframium" passed to an element from the previously known mineral wolframite, which was known as early as the 16th century, it was then called "wolf foam", or "Spuma lupi" in Latin, in German this phrase sounds like "Wolf Rahm" (Tungsten). The name was associated with the fact that tungsten, while accompanying tin ores, significantly interfered with the smelting of tin, because he transferred tin into a foam of slags (people began to say about this process: "Tin is devoured like a wolf a sheep!"). Currently in the USA, France, Great Britain and some other countries the name "tungsten" is used to name tungsten (from the Swedish tung sten, which translates as "heavy stone").
Tungsten is a gray, hard transition metal. The main application of tungsten is the role of the base in refractory materials in metallurgy. Tungsten is extremely refractory; under normal conditions, the metal is chemically resistant.
Tungsten differs from all other metals in unusual hardness, heaviness and refractoriness. Since ancient times, the people have used the expression "heavy as lead" or "heavier than lead", "leaden eyelids", etc. But it would be more correct to use the word "tungsten" in these allegories. The density of this metal is almost twice that of lead, to be precise, 1.7 times. With all this, the atomic mass of tungsten is lower and has a value of 184 versus 207 for lead.
Tungsten is a light gray metal with the highest melting and boiling points. Due to the plasticity and refractoriness of tungsten, it is possible to use it as filaments of lighting devices, in picture tubes, as well as in other vacuum tubes.
Twenty tungsten minerals are known. The most common: minerals of the scheelite group of wolframite, which are of industrial importance. Less common is wolframite sulfide, i.e. tungstensite (WS2) and oxide-like compounds - ferro- and cuprotungstite, tungstite, hydrotungstite. Vads, psilomelans with a high tungsten content are widespread.
Depending on the conditions of occurrence, morphology and type of tungsten deposits during their development, open, underground, and combined methods are used.
There are currently no methods for producing tungsten directly from concentrates. In this regard, intermediate compounds are first isolated from the concentrate, and then metallic tungsten is obtained from them. The separation of tungsten includes: decomposition of concentrates, then the transition of the metal into compounds, from which it is separated from the rest of its accompanying elements. Isolation of tungstic acid, i.e. pure chemical compound tungsten, continues with the subsequent production of tungsten in metallic form.
Tungsten is used in the manufacture of machinery and equipment for the metalworking, construction and mining industries, in the manufacture of lamps and lamps, in the transport and electronics industry, in the chemical industry and other areas.
Made of tungsten steel, the tool is able to withstand the enormous speeds of the most intense metalworking processes. Cutting speed with such a tool is usually measured in tens of meters per second.
Tungsten is rather poorly distributed in nature. The metal content in the earth's crust by weight is about 1.3 · 10 −4%. The main minerals containing tungsten are naturally occurring tungstates: scheelite, originally called tungsten, and wolframite.
Biological properties
The biological role of tungsten is negligible. Tungsten in its properties is very similar to molybdenum, but, unlike the latter, tungsten is not an essential element. Despite this fact, tungsten is quite capable of replacing molybdenum in animals and plants, in bacteria, while it inhibits the activity of Mo-dependent enzymes, for example, xanthine oxidase. Due to the accumulation of tungsten salts in animals, uric acid levels decrease and hypoxanthine and xanthine levels increase. Tungsten dust, like other metal dusts, is irritating to the respiratory system.
On average, about 0.001-0.015 milligrams of tungsten enters the human body per day with food. The assimilation of the element itself, like tungsten salts, in the human gastrointestinal tract is 1-10%, for poorly soluble tungstic acids - up to 20%. Tungsten mainly accumulates in bone tissue and kidneys. Bones contain about 0.00025 mg / kg, and human blood contains about 0.001 mg / L of tungsten. The metal is usually excreted from the body naturally in the urine. But 75% of the radioactive tungsten isotope 185W is excreted in the feces.
The dietary sources of tungsten, as well as its daily requirement, have not yet been studied. The toxic dose for the human body has not yet been identified. Death in rats occurs from a little more than 30 mg of the substance. In medicine, it is believed that tungsten does not have metabolic, carcinogenic and teratogenic effects on humans and animals.
Indicator of the elemental status of tungsten inside the human body: urine, whole blood. There are no data on a decrease in the level of tungsten in the blood.
An increased content of tungsten in the body most often occurs in workers of metallurgical plants engaged in the production of refractory and heat-resistant materials, alloy steels, as well as in people who come into contact with tungsten carbide.
The clinical syndrome "heavy metal disease" or pneumoconiosis can result from the chronic intake of tungsten dust into the body. Signs may include coughing, breathing problems, the development of atopic asthma, and changes in the lungs. The syndromes described above usually subside after prolonged rest, and simply in the absence of direct contact with vanadium. In the most severe cases, with delayed diagnosis of the disease, the pathology of cor pulmonale, emphysema and pulmonary fibrosis develops.
"Diseases of heavy metals" and the prerequisites for its occurrence usually appear as a result of exposure to several types of metals and salts (for example, cobalt, tungsten, etc.). It was found that the combined effect of tungsten and cobalt on the human body increases the detrimental effect on the pulmonary system. The combination of tungsten and cobalt carbides can cause local inflammation and contact dermatitis.
At the present stage of the development of medicine, there are no effective methods of accelerated metabolism or elimination of a group of metal compounds that can provoke the appearance of the "disease of heavy metals". That is why it is so important to constantly carry out preventive measures and timely identify people with high sensitivity to heavy metals, and carry out diagnostics at the initial stage of the disease. All these factors determine the further chances of success in the treatment of pathology. But in some cases, if necessary, therapy with complexing agents and symptomatic treatment are used.
More than half (or rather 58%) of all tungsten produced is used in the manufacture of tungsten carbide, and almost a quarter (more precisely, 23%) is used in the production of various steels and alloys. The production of tungsten "rolled products" (this includes filaments of incandescent lamps, electrical contacts, etc.) accounts for approximately 8% of the tungsten consumed in the world, and the remaining 9% is used to obtain catalysts and pigments.
Tungsten wire, which has found application in electric lamps, has recently acquired a new profile: it has been proposed to use it as a cutting tool in the processing of brittle materials.
The high strength and good ductility of tungsten make it possible to manufacture unique items from it. For example, a wire so thin can be drawn out of this metal that 100 km of this wire will have a mass of only 250 kg.
Molten liquid tungsten could remain in this state even near the surface of the Sun itself, because the boiling point of the metal is above 5500 ° C.
Many people know that bronze is composed of copper, zinc and tin. But, the so-called tungsten bronze is not only not bronze by definition, because does not contain any of the above-described metals, it is not an alloy at all, since purely metallic compounds are absent in it, and sodium and tungsten are oxidized.
Getting peach dye was very difficult, and often impossible at all. This is neither red nor pink, but some kind of intermediate, and even with a greenish tint. The lending says that more than 8000 attempts had to be used to obtain this paint. In the 17th century, only the most expensive porcelain items for the then Chinese emperor at a special factory in Shanxi province were decorated with peach paint. But when after some time it was possible to reveal the secret of a rare paint, it turned out that it was based on nothing more than tungsten oxide.
This happened in 1911. A student came to Yunnan Province from Beijing, his name was Li. Day after day he disappeared in the mountains, trying to find some kind of stone, as he explained, it was a pewter stone. But he didn’t succeed. The owner of the house where student Li lived lived with a young daughter named Xiao-mi. The girl was very sorry for the unlucky student and in the evening, during dinner, she told him simple, uncomplicated stories. One story told of an unusual stove, which was built from some kind of dark stones that fell straight from a cliff and laid in the backyard of their house. This stove turned out to be quite successful, and most importantly, it was durable; it served its owners for many years. Young Xiao-mi even presented the student with even one such stone. It was a brown stone, heavy as lead. Later it turned out that this stone was pure wolframite ...
In 1900, at the opening of the World Metallurgical Exhibition in Paris, completely new examples of high-speed steel (an alloy of steel with tungsten) were demonstrated for the first time. Literally immediately after this, tungsten began to be widely used in the metallurgical industry of all highly developed countries. But there is a rather interesting fact: for the first time tungsten steel was invented in Russia back in 1865 at the Motovilikh plant in the Urals.
In early 2010, an interesting artifact fell into the hands of Perm ufologists. It is believed to be a wreck of a spaceship. The analysis of the fragment showed that the object consists almost entirely of pure tungsten. Only 0.1% of the composition is accounted for by rare impurities. According to scientists, rocket nozzles are made from pure tungsten. But, so far it is not possible to explain one fact. In air, tungsten quickly oxidizes and rusts. But for some reason this piece does not corrode.
History
The word "tungsten" itself is of German origin. Previously, tungsten was not called the metal itself, but its main mineral, i.e. to wolframite. Some suggest that then this word was used almost as a swear word. From the early 16th to the second half of the 17th century, tungsten was considered a tin mineral. Although it really quite often accompanies tin ores. But from the ores, which included wolframite, much less tin was smelted. As if someone or something "devoured" the useful tin. This is where the name of the new element comes from. In German, Wolf (Wolf) means a wolf, and Ram (Ramm) in translation from ancient German means a ram. Those. the expression "eats tin like a wolf of a lamb" became the name of the metal.
The well-known chemical abstract journal of the United States or reference publications on all chemical elements of Mellor (England) and Pascal (France) do not even contain a mention of such an element as tungsten. Their chemical element number 74 is called tungsten. The symbol W, which denotes tungsten, has only gained widespread acceptance in the past few years. In France and Italy, until recently, the element was designated by the letters Tu, i.e. the first letters of the word tungstene.
The foundations for this confusion lie in the history of the discovery of the element. In 1783, the Spanish chemical scientists, the Eluard brothers, reported that they had succeeded in discovering a new chemical element. In the process of decomposition of the Saxon mineral "tungsten" with nitric acid, they managed to obtain "acidic earth", i.e. yellow precipitate of an oxide of unknown metal, the precipitate turned out to be soluble in ammonia. In the starting material, this oxide was together with oxides of manganese and iron. The Eluard brothers called this element tungsten, and the mineral from which the metal was mined wolframite.
But the Eluard brothers cannot be 100% called the discoverers of tungsten. Of course, they were the first to report their discovery in print, but ... In 1781, two years before the brothers' discovery, the famous Swedish chemist Karl Wilhelm Scheele found exactly the same “yellow earth” while treating another mineral with nitric acid. The scientist named it simply "tungsten" (translated from Swedish tung - heavy, sten - stone, ie "heavy stone"). Karl Wilhelm Scheele found that the "yellow earth" differs in its color, as well as in other properties, from the analogous molybdenum. The scientist also learned that in the mineral itself, it binds to calcium oxide. In honor of Scheele, the name of the mineral "tungsten" was changed to "sheelite". It is interesting that one of the Eluard brothers was a student of Scheele, in 1781 he worked in the teacher's laboratory. Neither Scheele nor the Eluard brothers began to share the discovery. Scheele simply did not claim this discovery, and the Eluard brothers did not insist on the priority of their primacy.
Many have heard of the so-called "tungsten bronzes". These are very beautiful metals in appearance. Blue tungsten bronze has the following composition Na2O · WO2 ·, and golden - 4WO3Na2O · WO2 · WO3; violet and purple-red occupy an intermediate position, in them the ratio of WO3 to WO2 is less than four, and more than one. As the formulas show, these substances contain neither tin, nor copper, nor zinc. These are not bronzes, and not alloys at all. they do not even have metallic compounds, and sodium and tungsten are oxidized here. Such "bronzes" resemble real bronze not only externally, but also by their properties: hardness, resistance to chemical reagents, high electrical conductivity.
In ancient times, the peach blossom was one of the rarest, it was said that it took 8000 experiments to get it. In the 17th century, the most expensive porcelain items of the Chinese emperor were painted in peach color. But after the disclosure of the secret of this paint, it suddenly turned out that the basis of it was tungsten oxide.
Being in nature
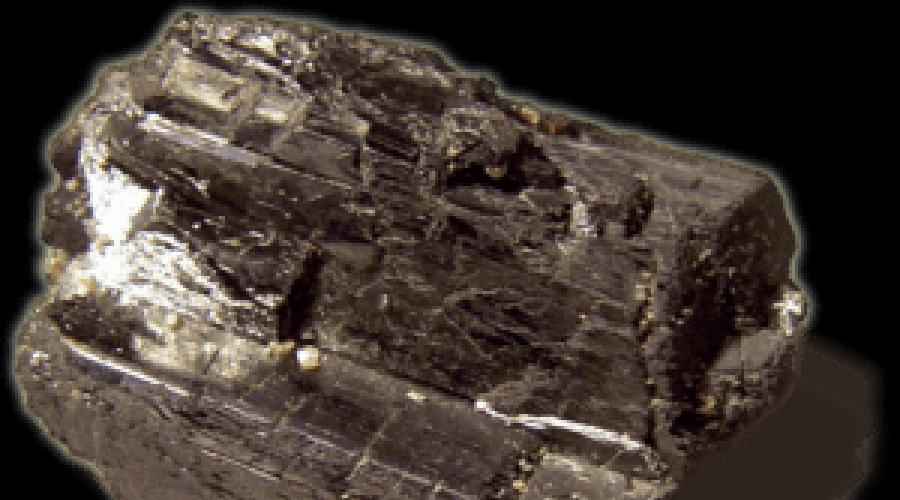
Tungsten is poorly distributed in nature, the metal content in the earth's crust is 1.3 · 10 -4% by weight. Tungsten is mainly found in the composition of complex oxidized compounds, which are formed by tungsten trioxide WO3, as well as oxides of iron and calcium or manganese, sometimes copper, lead, thorium and various rare earth elements. The most common mineral, wolframite, is a solid solution of tungstates, i.e. salts of tungstic acid, manganese and iron (nMnWO 4 mFeWO 4). The solution is in the form of hard and heavy crystals of black or brown color, depending on the predominance of various compounds in the composition of the solution. If there are more manganese compounds (Hübnerite), the crystals will be black, if iron compounds (ferberite) predominate, the solution will be brown. Wolframite is highly conductive and paramagnetic
As for other tungsten minerals, scheelite is of industrial importance, i.e. calcium tungstate (formula CaWO 4). The mineral forms lustrous crystals of light yellow and sometimes almost white colors. Scheelite is not magnetic at all, but it has another feature - the ability to luminescence. After ultraviolet light in the dark, it will fluoresce with a bright blue color. The presence of an impurity of molybdenum changes the color of the glow, it changes to pale blue, sometimes to cream. Thanks to this property, it is possible to easily detect geological deposits of the mineral.
Usually deposits of tungsten ore are associated with the area of distribution of granite. Large crystals of scheelite or wolframite are very rare. Usually minerals are simply embedded in granite rocks. It is rather difficult to extract tungsten from granite, because its concentration is usually no more than 2%. In total, no more than 20 tungsten minerals are known. Among them, stolzite and rasoite can be distinguished, which are two different crystalline modifications of lead tungstate PbWO 4. The rest of the minerals are decomposition products or secondary forms of common minerals, for example, scheelite and wolframite (hydrotungstite, which is a hydrated tungsten oxide, formed from wolframite; tungsten ocher), russelite, a mineral containing tungsten and bismuth oxides. The only non-oxide mineral of tungsten is tungstenite (WS 2), its main reserves are located in the USA. Typically, the tungsten content is in the range of 0.3% to 1.0% WO 3.
All tungsten deposits are of hydrothermal or magmatic origin. Scheelite and wolframite are quite often found in the form of veins, in places where magma has penetrated into cracks in the earth's crust. The main part of tungsten deposits is concentrated in areas of young mountain ranges - the Alps, Himalayas and the Pacific belt. The largest deposits of wolframite and scheelite are located in China, Burma, USA, Russia (Ural, Transbaikalia and Caucasus), Portugal and Bolivia. The annual production of tungsten ores in the world is approximately 5.95 · 104 tons of metal, of which 49.5 · 104 tons (or 83%) is extracted in China. In Russia, about 3400 tons are mined per year, in Canada - 3000 tons per year.
China plays the role of a global leader in the development of tungsten raw materials (the Jianshi deposit accounts for 60 percent of Chinese production, Hunan - 20 percent, Yunnan - 8 percent, Guandong - 6 percent, Inner Mongolia and Guanzhi - 2% each, there are others). In Russia, the largest deposits of tungsten ore are located in 2 regions: in the North Caucasus (Tyrnyauz, Kabardino-Balkaria) and in the Far East. The plant in Nalchik processes tungsten ore into ammonium paratungstate and tungsten oxide.
The largest consumer of tungsten is Western Europe (30%). USA and China - 25% each, 12% -13% - Japan. In the CIS, about 3000 tons of metal are consumed annually.
Application
In total, the world produces about 30 thousand tons of tungsten per year. Tungsten steel and other alloys containing tungsten and its carbides are used in the manufacture of tank armor, shells for shells and torpedoes, the most important parts of aircraft and internal combustion engines.
Tungsten is certainly present in the composition of the best types of tool steels. Metallurgy generally absorbs about 95% of all tungsten produced. As is typical for metallurgy, not only pure tungsten is used, mainly tungsten is used, which is cheaper - ferro-tungsten, i.e. an alloy with a tungsten content of about 80% and an iron content of about 20%. It is produced in electric arc furnaces.
Tungsten alloys have a number of remarkable properties. An alloy of tungsten, copper and nickel, as it is also called "heavy" metal, is a raw material for the manufacture of containers for storing radioactive substances. The protective effect of such an alloy is 40% higher than that of lead. This alloy is also used in radiotherapy, because with a relatively small screen thickness, sufficient protection is provided.
An alloy of tungsten carbide and 16% cobalt is so hard that it partially replaces diamond in well drilling. Pseudo alloys of tungsten with silver and copper are an excellent material for switches and switches in high voltage conditions. These products last 6 times longer than conventional copper contacts.
The use of pure tungsten or tungsten containing alloys is based in large part on their hardness, refractoriness and chemical resistance. Tungsten in its pure form is widely used in the production of filaments for electric lamps and cathode-ray tubes; it is used in the production of crucibles for the purpose of evaporating metals; it is used in the contacts of automobile ignition distributors; it is used in targets for X-ray tubes; It is used as a winding and heating element for electric furnaces, as well as a structural material for spacecraft and aircraft that operate at high temperatures.
Tungsten is a part of alloys of high-speed steels (tungsten content 17.5 - 18.5%), stellites (from cobalt with additions of Cr, C, W), hastalloy (stainless steels based on Ni), as well as many other alloys. Tungsten is used as a base in the production of heat-resistant and tool alloys, namely, ferro-tungsten (W 68–86%, Mo and iron up to 7%) is used, which is easily obtained by direct reduction of scheelite or wolframite concentrate. Tungsten is used in the production of Pobedit. It is a superhard alloy containing 80–85% tungsten, 7–14% cobalt, 5–6% carbon. Pobedit is simply irreplaceable in the metalworking process, as well as in the oil and mining industries.
Magnesium and calcium tungstates are widely used in fluorescent devices. Other tungsten salts are used in the tanning and chemical industries. Tungsten disulfide is a dry high-temperature grease, stable at temperatures up to 500 ° C. Tungsten bronzes and other tungsten compounds are used in the manufacture of paints. Quite a few tungsten compounds are excellent catalysts.
In the production of electric lamps, tungsten is indispensable because it is not only unusually refractory, but also quite plastic. 1 kg of tungsten is used as a raw material for the manufacture of 3.5 km of wire. Those. 1 kg of tungsten can be used to make filaments for 23 thousand 60-watt lamps. Thanks to this property alone, the electrical industry around the world consumes about one hundred tons of tungsten per year.
Production
The first stage in the production of tungsten is the enrichment of the ore, i.e. separation of valuable components from the main ore mass, waste rock. The same beneficiation methods are used as for other heavy metal ores: grinding and flotation, followed by magnetic separation (tungsten ores) and oxidative roasting. The concentrate obtained by this method is usually burned with an excess of soda, thereby bringing tungsten into a soluble state, i.e. into sodium wolframite.
Another method of obtaining this substance is leaching. Tungsten is extracted using a soda solution at elevated temperature and under pressure, followed by neutralization and precipitation of calcium tungstate, i.e. scheelite. Scheelite is obtained because it is quite easy to extract purified tungsten oxide from it.
CaWO 4 → H 2 WO 4 or (NH 4) 2 WO 4 → WO 3
Tungsten oxide is also obtained through chlorides. The tungsten concentrate is treated with chlorine gas at an elevated temperature. In this case, tungsten chlorides are formed, which are easily separated from other chlorides by sublimation. The resulting chloride can be used to obtain oxide or immediately extract metal from it.
In the next step, oxides and chlorides are converted to metallic tungsten. It is best to use hydrogen to reduce tungsten oxide. With this reduction, the metal is the purest. The reduction of the oxide takes place in a special tube furnace, where the "boat" with WO 3 moves through several temperature zones. Dry hydrogen flows towards the “boat”. Reduction of oxide occurs in hot (450-600 ° C) and cold zones (750-1100 ° C). In cold zones, reduction to WO 2 takes place, and then to metal. As time passes through the hot zone, the tungsten powder particles change their size.
Recovery can take place not only under hydrogen supply. Coal is often used. Due to the solid reducing agent, production is simplified, but the temperature in this case should reach 1300 ° C. The coal itself and the impurities that it always contains, entering into a reaction with tungsten, form carbides of other compounds. As a result, the metal becomes dirty. But only high quality tungsten is used in the electrical industry. Even 0.1% iron impurity is made by tungsten for making the thinnest wire, because it becomes much more fragile.
The separation of tungsten from chlorides is based on pyrolysis. Tungsten and chlorine form some compounds. An excess of chlorine allows all of them to be converted to WCl6, which in turn decomposes into chlorine and tungsten at a temperature of 1600 ° C. If hydrogen is present, the process starts at 1000 ° C.
This is how tungsten is obtained in the form of a powder, which is then pressed at a high temperature in a stream of hydrogen. The first stage of pressing (heating to about 1100-1300 ° C) produces a brittle porous ingot. Then pressing continues, and the temperature begins to rise almost to the melting point of tungsten. In such an environment, the metal begins to become solid and gradually acquires its qualities and properties.
On average, 30% of the tungsten produced in the industry is tungsten from recycled materials. Tungsten scrap, sawdust, shavings and powder are oxidized and converted into ammonium paratungstate. As a rule, scrap of cutting steels is disposed of at the enterprise that produces the same steels. Scrap from electrodes, incandescent lamps and chemical reagents is almost never recycled anywhere.
In the Russian Federation, tungsten products are produced at: Skopinsky Hydrometallurgical Plant Metallurg, Vladikavkaz Plant Pobedit, Nalchik Hydrometallurgical Plant, Kirovgrad Hard Alloys Plant, Elektrostal, Chelyabinsk Electrometallurgical Plant.
Physical properties
Tungsten is a light gray metal. It has the highest melting point of any known element except carbon. The value of this indicator is approximately from 3387 to 3422 degrees Celsius. Tungsten has excellent mechanical properties when reaching high temperatures; among all metals, tungsten has the lowest value of such an indicator as the coefficient of expansion.
Tungsten is one of the heaviest metals with a density of 19,250 kg / m3. The metal has a cubic body-centered lattice with a parameter a = 0.31589 nm. At a temperature of 0 degrees Celsius, the electrical conductivity of tungsten is only 28% of the value of the same indicator for silver (silver - conducts current better than any other metal). Pure tungsten is very easy to process, but in its pure form it is rare, more often it has impurities of carbon and oxygen, due to which it gets its well-known hardness. The electrical resistance of the metal at a temperature of 20 degrees Celsius is 5.5 * 10 -4, at a temperature of 2700 degrees Celsius - 90.4 * 10 -4.
Tungsten differs from all other metals in its special refractoriness, heaviness and hardness. The density of this metal is almost twice that of the same lead, or rather 1.7 times. But the atomic mass of the element, on the contrary, is lower and is 184 versus 207.
The values of the tensile and compressive moduli of tungsten are unusually high, a huge resistance to thermal creep, the metal has high electrical and thermal conductivity. Tungsten has a fairly high coefficient of electron emission, which can be significantly improved by fusing the element with oxides of some other metals.
The color of the resulting tungsten depends to a large extent on the method of its production. Fused tungsten is a shiny gray metal that looks a lot like platinum in appearance. Tungsten powder can be gray, dark gray and even black: the smaller the grains of the powder, the darker it will be.
Tungsten is highly durable: at room temperature, it does not change in air; upon reaching the red-hot temperature, the metal begins to slowly oxidize, releasing tungstic acid anhydride. Tungsten is almost insoluble in sulfuric, hydrofluoric and hydrochloric acids. In aqua regia and nitric acid, the metal is oxidized from the surface. Being in a mixture of hydrofluoric and nitric acid, tungsten dissolves, thus forming tungstic acid. Of all the tungsten compounds, the most practical benefits are: tungsten anhydride or tungsten trioxide, peroxides with the general formula ME2WOX, tungsten compounds, compounds with carbon, sulfur and halogens.
Tungsten, which occurs in nature, consists of 5 stable isotopes whose mass numbers are 186,184, 183, 182, 181. The most common isotope with a mass number of 184, its share is 30.64%. Of the whole relative set of artificial radioactive isotopes of element 74, only three are of practical importance: tungsten-181 (its half-life is 145 days), tungsten-185 (its half-life is 74.5 days), tungsten-187 (its half-life is half-life is 23.85 hours). All these isotopes are formed inside nuclear reactors in the process of bombarding tungsten isotopes with neutrons from a natural mixture.
The valence of tungsten has a changeable character - from 2 to 6, the most stable hexavalent tungsten, the trivalent and divalent compounds of a chemical element are unstable and have no practical value. The radius of the tungsten atom is 0.141 nm.
Clarke of tungsten of the earth's crust according to Vinogradov is 0.00013 g / t. Its average content in the composition of rocks, gram / ton: ultrabasic - 0.00001, basic - 0.00007, medium - 0.00012, acidic - 0.00019.
Chemical properties
Tungsten is not affected by: aqua regia, sulfuric, hydrochloric, hydrofluoric and nitric acids, aqueous sodium hydroxide solution, mercury, mercury vapor, ammonia (up to 700 ° C), air and oxygen (up to 400 ° C), hydrogen, water, hydrogen chloride (up to 600 ° C), carbon monoxide (up to 800 ° C), nitrogen.
After a little heating, dry fluorine begins to combine with finely ground tungsten. As a result, hexafluoride (formula WF 6) is formed - this is a very interesting substance that has a melting point of 2.5 ° C and a boiling point of 19.5 ° C. After reaction with chlorine, a similar compound is formed, but the reaction is possible only at a temperature of 600 ° C. WC16, a steel blue crystal, begins to melt at 275 ° C and boil at 347 ° C. Tungsten forms weakly stable compounds with iodine and bromine: tetra- and diiodide, penta- and dibromide.
At high temperatures, tungsten can combine with selenium, sulfur, nitrogen, boron, tellurium, silicon, and carbon. Some of these compounds are surprisingly hard, as well as other excellent qualities.
Of particular interest is the carbonyl (formula W (CO) 6). Tungsten here combines with carbon monoxide, and, therefore, has zero valence. Tungsten carbonyl is produced under special conditions, because it is extremely unstable. At 0 ° C, it separates from a special solution in the form of colorless crystals, after reaching 50 ° C, carbonyl sublimes, at 100 ° C it completely decomposes. But it is thanks to this compound that dense and hard tungsten coatings (from pure tungsten) can be obtained. Many tungsten compounds, like tungsten itself, are very active. For example, tungsten oxide tungsten oxide WO 3 has the ability to polymerize. In this case, the so-called heteropoly compounds (their molecules can contain more than 50 atoms) and isopoly compounds are formed.
Tungsten (VI) oxide WO 3 is a crystalline substance that has a light yellow color, turns orange when heated. The oxide has a melting point of 1473 ° C and a boiling point of 1800 ° C. The tungstic acid corresponding to it is not stable, in a solution of water the dihydrate precipitates, while it loses one molecule of water at a temperature of 70 to 100 ° C, and the second molecule at a temperature of 180 to 350 ° C.
Anions of tungstic acids are prone to the formation of polycompounds. As a result of the reaction with concentrated acids, mixed anhydrides are formed:
12WO 3 + H 3 PO 4 = H 3.
As a result of the reaction of tungsten oxide and metallic sodium, non-stoichiometric sodium tungstate is obtained, which is called "tungsten bronze":
WO 3 + xNa = Na x WO 3.
In the process of reduction of tungsten oxide with hydrogen, during the separation, hydrated oxides with a mixed oxidation state are obtained, they are called "tungsten blue":
WO 3 – n (OH) n, n = 0.5–0.1.
WO 3 + Zn + HCl = ("blue"), W 2 O 5 (OH) (brown)
Tungsten (VI) oxide is an intermediate product in the production process of tungsten, as well as its compounds. It is a component of individual ceramic pigments and industrially important hydrogenation catalysts.
WCl 6 - Higher tungsten chloride, formed as a result of the interaction of metallic tungsten or tungsten oxide with chlorine, fluorine, or carbon tetrachloride. After reduction of tungsten chloride with aluminum, tungsten carbonyl is formed together with carbon monoxide:
WCl 6 + 2Al + 6CO = + 2AlCl 3 (on air)